
A Short History of Nearly Everything
Bill Bryson
 GENRE: History
GENRE: History
 PAGES: 544
PAGES: 544
 COMPLETED: June 29, 2024
COMPLETED: June 29, 2024
 RATING:
RATING: 




Short Summary
Drawing on three years of intensive study and interviews with experts around the world, Bill Bryson delivers a wide-sweeping and entertaining history of Earth. Among many topics, A Short History of Nearly Everything delves into the Big Bang, our solar system, and the evolution of life on Earth.
Key Takeaways



Favorite Quote
“We [humans] are so used to the notion of our own inevitability as life's dominant species that it is hard to grasp that we are here only because of timely extraterrestrial bangs and other random flukes. The one thing we have in common with all other living things is that for nearly four billion years our ancestors have managed to slip through a series of closing doors every time we needed them to.”
Book Notes 
Introduction
- About the Author — Bill Bryson is an author and journalist who has written several best-selling books, including this one and The Body. He was born in Des Moines, Iowa before moving to the United Kingdom, where he has lived most of his life.
- About the Book — Wanting to learn more about the world we live in, Bryson spent three years studying science and the Earth. How did our universe come to be? What is Earth made of? These are a few of the many questions Bryson tackled in his studies. This book is the result of his three years of reading books and journals, and interviewing scientists around the world.
- Everything Is Made of Atoms — Our physical bodies are made of trillions of atoms, and they assemble together miraculously to form us. The atoms we are made of are pretty mundane: carbon, hydrogen, oxygen, nitrogen, a little bit of calcium, a splash of sulfur, and a few other ordinary elements. When we die, the atoms that made us drift off and become something else. Everything in life is made of atoms.
- Quote (P. 2): “Even a long human life adds up to only about 650,000 hours. And when that modest milestone flashes past or at some other point thereabouts, for reasons unknown your atoms will shut you down silently disassemble, and go off to be other things. And that’s it for you.”
- Quote (P. 2): “Whatever else it may be, at the level of chemistry life is curiously mundane: carbon, hydrogen, oxygen and nitrogen, a little calcium, a dash of sulfur, a light dusting of other very ordinary elements — nothing you wouldn’t find in any ordinary drugstore — and that’s all you need. The only thing special about the atoms that make you is that they make you. That is of course the miracle of life”
- Quote (P. 2): “Whether or not atoms make life in other corners of the universe, they make plenty else; indeed, they make everything else. Without them there would be no water or air or rocks, no stars and planets, no distant gassy clouds or swirling nebulae or any of the other things that make the universe so usefully material.”
- Interesting Fact — Survival on Earth has been tricky business when you look at the 4.5 billion years it’s been around. About 99.9% of all living things that have ever existed on Earth are dead. The average species only lasts around 4 million years or so on this planet. It’s pretty miraculous that any living thing that is here today has evolved successfully over the last 3.8 billion years (when life first began on Earth) without becoming extinct.
- Quote (P. 3): “Of the billions and billions of species of living things that have existed since the dawn of time, most — 99.99 percent — are no longer around. . . The average species on Earth lasts for only about four million years, so if you wish to be around for billions of years, you must be as fickle as the atoms that made you.”
- We Are Lucky — The fact that we are here today is truly miraculous. In order to be here today, every one of us had to have ancestors and forbears on both sides of the family who — over the span of 4.5 billion years Earth has been around — survived without being eaten or killed, were attractive enough to find partners, and healthy enough to reproduce. Life is a blessing.
- Quote (P. 3): “Consider the fact that for 3.8 billion years, a period of time older than the Earth’s mountains and rivers and oceans, every one of your forebears on both sides has been attractive enough to find a mate, healthy enough to reproduce, and sufficiently blessed by fate and circumstances to live long enough to do so. Not one of your pertinent ancestors was squashed, devoured, drowned, starved, stranded, stuck fast, untimely wounded, or otherwise deflected from its life’s quest of delivering a tiny charge of genetic material to the right partner at the right moment in order to perpetuate the only possible sequence of hereditary combinations that could result — eventually, astoundingly, and all too briefly — in you.”
- Quote (P. 349): “We [humans] are so used to the notion of our own inevitability as life’s dominant species that it is hard to grasp that we are here only because of timely extraterrestrial bangs and other random flukes. The one thing we have in common with all other living things is that for nearly four billion years our ancestors have managed to slip through a series of closing doors every time we needed them to.”
Ch. 1: How to Build a Universe
- The Big Bang — For a very long time, absolutely nothing existed. There were no atoms and no universe for them to float around in. There was nothing. Then something happened — what scientists have called the Big Bang. Essentially, the universe was created when every speck of its energy was jammed into a very tiny point. This extremely dense point exploded with unimaginable force, creating matter and propelling it outward to make the billions of galaxies of our vast universe. Astrophysicists have called this titanic explosion the Big Bang, and it all happened in about three minutes. So, from literally nothing, our universe began. When did this all happen? There’s a lot of debate, but scientists seem to be heading towards a consensus of 13.7 billion years ago.
- Quote (P. 10): “In a single blinding pulse, a moment of glory much too swift and expansive for any form of words, the singularity assumes heavenly dimensions, space beyond conception. In the first lively second (a second that many cosmologists will devote careers to shaving into ever-finer wafers) is produced gravity and the other forces that govern physics. In less than a minute the universe is a million billion miles across and growing fast. There is a lot of heat now, ten billion degrees of it, enough to begin the nuclear reactions that create the lighter elements — principally hydrogen and helium, with a dash (about one atom in a hundred million) of lithium. In three minutes, 98 percent of all the matter there is or will ever be has been produced. We have a universe.”
- Quote (P. 13): “Although everyone calls it the Big Bang, many books caution us not to think of it as an explosion in the conventional sense. It was, rather, a vast, sudden expansion on a whopping scale.”
- Big Bang Static — In 1965, astronomers Arno Penzias and Robert Wilson began to pick up a weird static noise in the cosmos while trying to use a large communications antenna owned by Bell Laboratories in New Jersey. For a year, they tried to get rid of this sound because it was negatively affecting the work they were trying to do. What they didn’t know at the time was that they were picking up cosmic radiation left over from the Big Bang that was coming from the edge of our universe, nearly 90 billion trillion miles away. Once they figured out what they were hearing, they posted several papers about it and were awarded the Nobel Prize in Physics in 1978. This finding expanded our knowledge of the sheer depth of our universe — at the time, we only knew about a few galaxies. This finding helped us understand that there was even more space in the universe beyond what we previously knew about.
- Quote (P. 12): “In his book The Inflationary Universe, Alan Guth provides an analogy that helps to put this finding in perspective. If you think of peering into the depths of the universe as like looking down from the hundredth floor of the Empire State Building (with the hundredth floor representing now and street level representing the moment of the Big Bang), at the time of Wilson and Penzias’s discovery the most distant galaxies anyone had ever detected were on about the sixtieth floor, and the most distant things — quasars — were on about the twentieth. Penzias and Wilson’s finding pushed our acquaintance with the visible universe to within half an inch of the sidewalk.”
- Inflation Theory — Inflation theory was first propounded in 1979 by Alan Guth. This theory helps explain what happened milliseconds after the Big Bang. Essentially, instantly after the Big Bang, our universe expanded (or inflated) at least a hundred billion light years across and created 98% of everything that makes up our universe. According to this theory, gravity emerged first, followed by nuclear forces and elementary particles.
- Quote (P. 14): “The eventual result was the inflation theory, which holds that a fraction of a moment after the dawn of creation [the Big Bang], the universe underwent a sudden dramatic expansion. It inflated — in effect ran away with itself, doubling in size every 10 seconds. . . it changed the universe from something you could hold in your hand to something at least 10,000,000,000,000,000,000,000,000 times bigger.”
- Quote (P. 15): “It is enough to know that in a single cracking instant we were endowed with a universe that was vast — at least a hundred billion light-years across, according to the theory, but possibly any size up to infinite — and perfectly arrayed for the creation of stars, galaxies, and other complex systems.”
- Space Curves — Our universe is one of many universes. If you were to travel to the edge of our universe, which is impossible as it is billions of light years away, you would never arrive at a set “boundary.” Instead, as soon as you stepped over the edge, you would end up right back where you started. That’s because space curves in a way that allows it to be boundless; we are not in some bubble.
- How Did We Get Here? — What’s interesting about the Big Bang is that it created 98% of everything (matter) that exists, but that matter consisted only of light gases such as helium, hydrogen, and lithium. The heavy gases that are so essential for humans and other organisms — gases such as carbon, nitrogen, and oxygen — were not created when this Big Bang occurred. So how did we get here? Later chapters in this book will address this question.
- Quote (P. 18): “For a long time the Big Bang theory had one gaping hole that troubled a lot of people — namely that it couldn’t begin to explain how we got here. Although 98 percent of all the matter that exists was created with the Big Bang, that matter consisted exclusively of light gases: the helium, hydrogen, and lithium that we mentioned earlier. Not one particle of the heavy stuff so vital to our own being — carbon, nitrogen, oxygen, and all the rest — emerged from the gaseous brew of creation. But — and here’s the troubling point — to forge these heavy elements, you need the kind of heat and energy of a Big Bang. Yet there has been only one Big Bang and it didn’t produce them. So where did they come from?”
- Chapter Takeaway — Our universe emerged following a Big Bang, which launched a massive expansion (or inflation) that created 98% of the matter involved in our world. Prior to this Big Bang, nothing existed. Interestingly, none of the heavy gases that are essential to life forms — carbon, nitrogen, oxygen — were created during this Big Bang.
Ch. 2: Welcome to the Solar System
- Our Solar System — Our solar system originated about 4.6 billion years ago, and Earth was one of the first planets to be born in it (about 4.5 billion years ago). Our solar system consists of eight planets: Earth, Jupiter, Saturn, Mars, Neptune, Venus, Mercury, and Uranus. Pluto was announced as a planet in 1999, but in 2006 was relegated to a “dwarf planet.” We have four rocky inner planets (Mercury, Venus, Earth, and Mars) and four gassy outer giants (Jupiter, Saturn, Uranus, and Neptune). Gravity holds all of these planets in orbit around the Sun. When it was considered a planet, Pluto was the tiniest of them all, and it was so far away from the sun that it was basically an ice ball (the Sun was so far away from Pluto that it appears as the size of a tiny star if you were standing on it).
- More About Our Solar System — We also have something called an Oort Cloud far beyond Pluto that is home to many comets. The Oort Cloud is the furthest region in our solar system. Occasionally, a comet in the Oort Cloud can get knocked off its orbit slightly, sending it into orbit around the Sun. This happens about 3-4 times per year, and these comets become known as “long-period comets.” Astronomers have discovered at least 290 moons orbiting the planets in our solar system. Earth only has one, but Saturn has 146. There are between 100-400 billion stars in the Milky Way, and the Milky Way is one of 140 billion galaxies, many of which are larger than ours. The closest star to us is called Proxima Centauri, and it is 4.2 light years away from the Sun, which is 100 million times farther than a trip to the moon. Space is enormous.
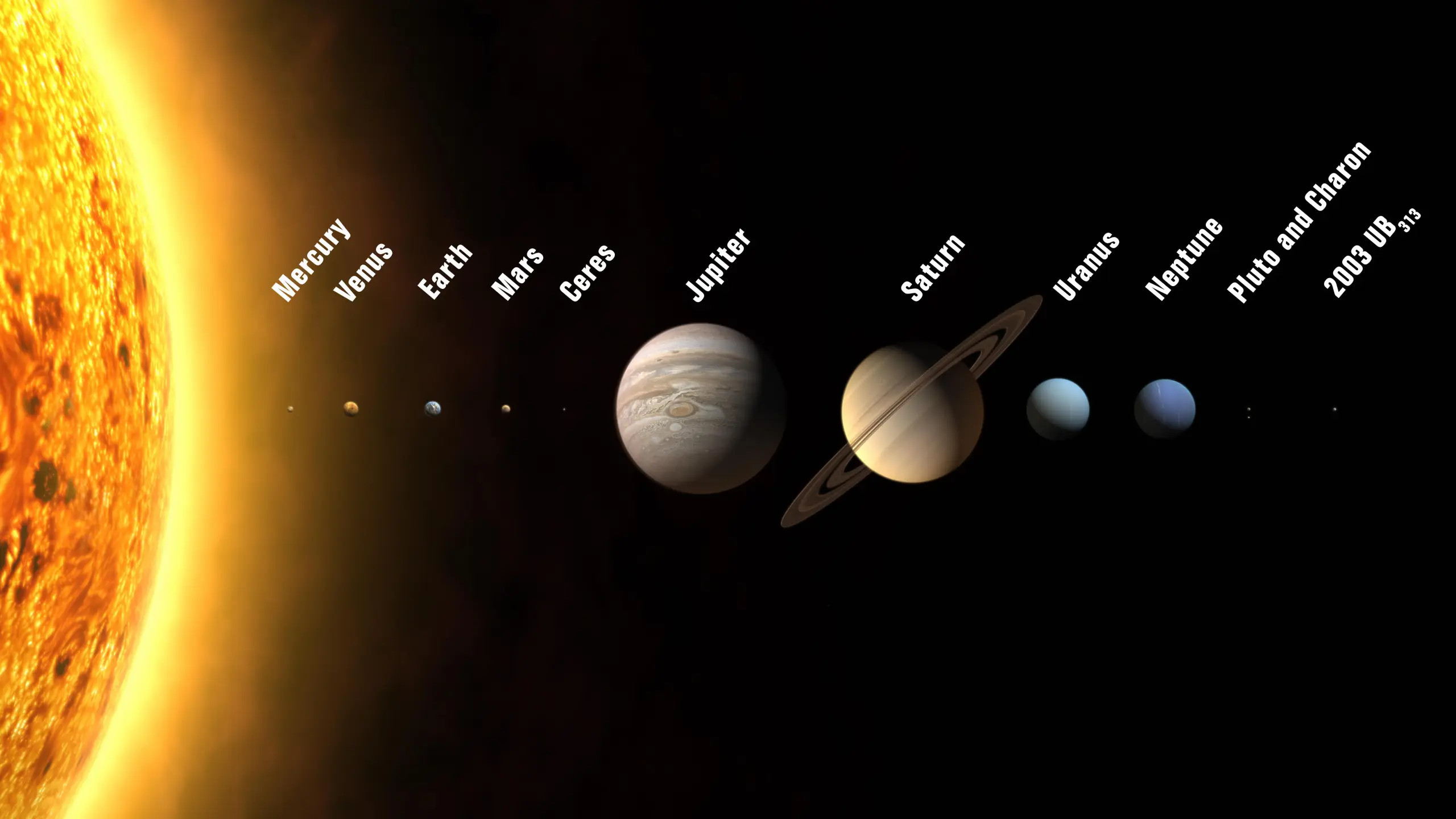
- Space Is Enormous — Space is absolutely enormous. It’s not even really possible to comprehend just how enormous it is. Our entire solar system — including the Sun, Earth, Oort Cloud, other planets, and everything else — fills a tiny fraction of the available space. We are literally a speck of dust in the grand scheme of space.
- Quote (P. 24): “Our solar system may be the liveliest thing for trillions of miles, but all the visible stuff in it — the Sun, the planets and their moons, the billion or so tumbling rocks of the asteroid belt, comets, and other miscellaneous drifting detritus — fills less than a trillionth of the available space.”
- Quote (P. 27): “Space, let me repeat, is enormous. The average distance between stars out there is 20 million million miles. Even at speeds approaching those of light, these are fantastically challenging distances for any traveling individual.”
- Chapter Takeaway — Space is absolutely enormous, and our solar system is a speck of dust inside it.
Ch. 3: The Reverend Evans's Universe
- Light From Stars — When you look up at the sky and spot stars, the light you’re seeing is likely not light from the star as it is today but rather it’s really a history of that star. It’s entirely possible that the star is dead, and the light emitted from it hasn’t yet traveled far enough to burn out. The light from fallen stars takes a very long time to burn out.
- Quote (P. 29): “Glance at the night sky and what you see is history and lots of it — the stars not as they are now but as they were when their light left them. For all we know, the North Star, our faithful companion, might actually have burned out last January or in 1854 or at any time since the early fourteenth century and news of it just hasn’t reached us yet. The best we can say — can ever say — is that it was still burning on this date 680 years ago.”
- Supernova — A supernova is a reaction that occurs when a giant star, one much bigger than our own Sun, collapses and then explodes, releasing in an instant the energy of a hundred billion suns, burning brighter than all the stars in its galaxy. A supernova is essentially like a trillion hydrogen bombs going off at once. When atoms in a really dense star are crushed together, electrons are forced into the nucleus to form neutrons. When this happens, it becomes a neutron star. The core of a neutron star is so dense that a spoonful matter from it would weigh 200 billion pounds. Now, when a neutron star collapses, there is a huge amount of energy that is released — this reaction is a supernova. They are huge explosions. Not all stars die this way; some fizzle out peacefully. In fact, in a typical galaxy consisting of a hundred billion stars, a supernova will occur only about once every 300 years. The amazing thing is that it takes millions of years for the light from a supernova to reach Earth, so anybody who spots one is seeing light from an explosion that happened a very long time ago.
- Magnetosphere — The magnetosphere is a magnetic zone high above Earth that protects us from ultraviolet rays and other cosmic things. Without our magnetosphere, we would get cooked by the Sun. We would not be able to live on this planet without it. That said, if a supernova ever occurred close enough to us, it would blow right through our magnetosphere and we’d all be dead.
- Supernovas: Why We’re Here — As mentioned in a previous chapter, the Big Bang that created our universe 13.8 billion years ago did not produce some of the heavy gases — such as carbon, nitrogen, and oxygen — that we on Earth need to survive. So what did? Supernovas are the answer. These explosions were so hot that they forged carbon and iron and the other heavy elements we need in a process known as nucleosynthesis. The explosions of these stars (which are dozens of times bigger than our Sun) sprayed these heavy elements into the cosmos where they formed gaseous clouds that eventually led to the creation of our solar system 4.6 billion years ago. About 99% of this gas went toward creating the Sun. Tiny fragments of the leftover material did, however, collide to form the Earth. With young Earth established, we were later hit by something huge that blew out enough material from our crust to create the Moon. As the Earth continued to grow over the next millions of years, an atmosphere was formed. We continued to get hit by comets, meteors, and other debris, which brought water to fill our oceans and other things we need for life on this planet.
- Quote (P. 38): “According to Hoyle’s theory, an exploding star would generate enough heat to create all the new elements and spray them into the cosmos where they would form gaseous clouds — the interstellar medium as it is known — that could eventually coalesce into new solar systems. With the new theories it became possible at last to construct plausible scenarios for how we got here. . . About 4.6 billion years ago, a great swirl of gas and dust some 15 billion miles across accumulated in space where we are now and began to aggregate. Virtually all of it — 99.9 percent of the mass of the solar system — went to make the Sun. Out of the floating material that was left over, two microscopic grains floated close enough together to be joined by electrostatic forces. This was the moment of conception for our planet.”
- Quote (P. 38): “In just 200 million years, possibly less, the Earth was essentially formed, though still molten and subject to constant bombardment from all the debris that remained floating about. At this point, about 4.5 billion years ago, an object the size of Mars crashed into Earth, blowing out enough material to form a companion sphere, the Moon. . . When Earth was only about a third of its eventual size, it was probably already beginning to form an atmosphere, mostly of carbon dioxide, nitrogen, methane, and sulfur. . . For the next 500 million years the young Earth continued to be pelted relentlessly by comets, meteorites, and other galactic debris, which brought water to fill the oceans and the components necessary for the successful formation of life.”
- Chapter Takeaway — Supernovas are giant explosions that occur when a neutron star collapses. These explosions are so hot that they created the heavy elements that eventually led to the birth of our solar system and Earth 4.5 billion years ago. The universe itself was born by the Big Bang 13.8 billion years ago. Supernovas are a big reason why we exist.
Ch. 4: The Measure of Things
- Isaac Newton & Principia — In 1687, Isaac Newton published his masterpiece Principia, which contained his three laws of motion (which state that a thing moves in the direction in which it is pushed; that it will keep moving in a straight line until some other force acts to slow or deflect it; and that every action has an equal and opposite reaction). It also contained his universal law of gravitation. The book launched Newton to worldwide fame. His laws explained so many things — the slosh and roll of ocean tides, the orbital motion of planets, why cannonballs trace a particular trajectory before crashing back down to Earth, why we aren’t sent into space as the planet spins and rotates, and much more.
- Quote (P. 48): “[Newton’s law of gravitation] states that every object in the universe exerts a tug on every other. It may not seem like it, but as you sit here now you are pulling everything around you — walls, ceiling, lamp, pet cat — toward you with your own little (indeed, very little) gravitational field. And these things are also pulling on you. It was Newton who realized that the pull of any two objects is, to quote Feynman again, ‘proportional to the mass of each and varies inversely as the square of the distance between them.’ Put another way, if you double the distance between two objects, the attraction between them becomes four times weaker.”
- Quote (P. 49): “[Newton’s law of gravitation] was the first really universal law of nature ever propounded by a human mind, which is why Newton is regarded with such universal esteem.”
- Knowing Earth — Earth’s weight is 5.9725 billion trillion metric tons. Earth’s median distance to the Sun is 149.59 million kilometers. Scientists in the 1700s went through some pretty wild things in order to complete their calculations and arrive at these numbers.
Ch. 5: The Stone-Breakers
- Lord Kelvin — Lord Kelvin, who was known by William Thomson for much of his life, had a heck of a career. Born in 1824, he quickly became a prodigy in his early years. In the course of a long career, he wrote 661 papers, accumulated 69 patents (which made him rich), and made an impact in nearly every branch of the physical sciences. Among many things, he suggested the method that led directly to the invention of refrigeration, devised the scale of absolute temperature that still bears his name (the Kelvin), and made many improvements to shipping and navigation, from the invention of a popular marine compass to the creation of the first depth sounder. His theoretical work, in electromagnetism, thermodynamics, and the wave theory of light, was equally revolutionary.
Ch. 6: Science Red in Tooth and Claw
- Extinction — In 1796 Georges Cuvier wrote a landmark paper, Note on the Species of Living and Fossil Elephants, in which he put forward for the first time a formal theory of extinctions. His belief was that from time to time the Earth experienced global catastrophes in which groups of creatures were wiped out. At the time, nobody knew how old the Earth was, so the idea of creatures living at one time and later becoming extinct was one that drew a lot of attention.
- Fossil Hunters — The 19th century was one in which the world became fascinated by fossils. During this time, fossil hunters were finding the remains of strange creatures all over the place. Mary Anning was one such fossil hunter. In 1812, she found a strange fossilized sea monster, 17 feet long and now known as the ichthyosaurus, embedded in the steep cliffs along the English Channel. Anning also later found the first plesiosaurus, another marine monster. The fossils of this creature took her 10 years to excavate. Although these weren’t technically dinosaurs, they were evidence that very strange creatures used to roam Earth millions and millions of years ago.
- Dinosaurs — In the 19th century, dinosaurs had not really been “discovered” yet, but their fossils were popping up all over. William Buckland was technically the first person to write a full account of a fossil dinosaur when he penned a report in 1824 detailing a dinosaur that he found near Oxford. He called it The Megalosaurus, meaning “big lizard.” The name comes from the fact that he originally thought the fossils were those of a lizard. Megalosaurus lived in the Middle Jurassic period, between 165 to 168 million years ago. In 1841, Richard Owen coined the term dinosauria, meaning “terrible lizard.” This was the first time these creatures were officially named and identified as dinosaurs. Owen himself found the fossils of many different dinosaurs during his great career. Meanwhile, in the U.S., Edward Cope and Othaniel Marsh helped expand the number of known dinosaur species in America from 9 to 150. Among many, they discovered now popular dinosaurs like the stegosaurus, triceratops, and more.
Ch. 7: Elemental Matters
- Phosphorous & Urine — In 1675, Hennig Brand became convinced, hilariously, that gold could somehow be distilled from human urine. The similarity of color seemed to be a factor in his thinking. He assembled 50 buckets of human urine in his cellar. Using various chemical techniques, he then converted the urine into paste and then into a waxy substance. None of this yielded gold, obviously, but he did find that the substance would glow and, when exposed to air, it sometimes burst into flames. This substance had huge commercial potential and soon became known as phosphorus. At first, soldiers would pee in buckets to provide the raw material to make phosphorus, but in the 1750s Swedish chemists figured out how to manufacture the chemical in bulk without urine. This mastery of phosphorous is why Sweden continues to be a leading producer of matches. Phosphorous is found in the head of matches, allowing them to ignite when struck.
- The Conservation of Mass — In the 1780s, Laurent Lavoisier found that a rusting object does not lose weight as everyone had assumed; rather it gains weight. Somehow the rusting object was attracting elemental particles from the air. This was a big discovery at the time because it was the first realization that matter can be transformed but not eliminated. This is known as the conservation of mass.
- Quote (P. 101): “If you burned this book now, its matter would be changed to ash and smoke, but the net amount of stuff in the universe would be the same. This became known as the conservation of mass, and it was a revolutionary concept.”
- Fun With Nitrous Oxide — In the early 1800s, people in England enjoyed inhaling nitrous oxide, or laughing gas, after it was discovered that it delivered a “highly pleasurable feeling.” This behavior continued for nearly 50 years. In 1846, scientists finally figured out that this gas could be used to great effect as an anesthetic in the operating room. Prior to nitrous oxide being used in the operating room, people had to undergo surgery without any type of numbing or pain agent. Meanwhile, people were using the stuff to produce laughing fits.
- The Periodic Table — Dmitri Mendeleyev created the periodic table of elements in the early 1870s. At the time, the organization of known elements (metals and gases) was very discombobulated. Inspired by the layout of the game solitaire, Mendeleyev combined metals and gases into one table, placing his elements into groups of 7. He arranged the elements in horizontal rows called periods and vertical columns called groups. This instantly showed one set of relationships when read up and down and another when read side to side. Because the properties repeated themselves periodically, the table was named the periodic table. The periodic table was a massive development that significantly helped advance the science of chemistry.
- Hydrogen & Helium — Today, we have about 120 known elements — 92 naturally occurring ones and a couple dozen that have been created in labs. Hydrogen is the most common element in the universe. Helium, the second most abundant element, was first discovered not on Earth but in the Sun, where it was found with a spectroscope during a solar eclipse, which is why the name (helium) honors the Greek sun god Helios. It wouldn’t be isolated until 1895.
- Radioactivity — In 1896, Henri Becquerel left a packet of uranium salts on a photographic plate. When he looked at the plate some time later, the salts had burned an impression in the plate, just as if the plate had been exposed to light. Scientists later discovered that certain kinds of rocks poured out constant and huge amounts of energy without diminishing in size. Marie Curie called this “radioactivity.” Curie is the only person ever to win a Nobel Prize in chemistry and physics. Scientists also found that these small radioactive materials have huge amounts of reserve energy stored inside them, and that the radioactive decay of these reserves could amount for the Earth’s warmth. This helped them narrow down the age of Earth. They also found that radioactive elements decayed into other elements — one day you could have an atom of uranium and the next you could have an atom of lead. This was extraordinary — it was pure alchemy and nobody thought that something like this could occur naturally.
Ch. 8: Einstein's Universe
- Quantum Theory — In 1900, Max Planck at the University of Berlin unveiled a new quantum theory, which posited that energy is not a continuous thing like flowing water but comes in individual packets, which he called quanta. This was a novel concept because it demonstrated that light didn’t need to be a wave as was previously believed. More importantly, it laid the foundation for Albert Einstein and modern physics. Quantum theory was really the first clue that the world was about to change.
- Albert Einstein — Einstein was born in Ulm (Southern Germany) in 1879 but grew up in Munich. Nothing about his childhood indicted that he would become a genius. Famously, he didn’t learn to speak until he was three years old. When he moved to Switzerland to continue his education as a teenager, he failed his entrance exams. He later studied at the Zurich Polytechnic Institute, where he graduated in 1900. He went on to have a child out of wedlock around the same time. The child was secretly put up for adoption, and Einstein never saw her. In 1902, Einstein took a job with the Swiss patent office, where he stayed for the next seven years. The job kept him busy while also allowing him to focus on his physics in off hours. Einstein, humorously, had a really hard time getting a teaching job early in his career. He applied to many and was always rejected, even after his ‘miracle year’ of 1905.
- Einstein: Miracle Year — In one remarkable year, 1905, Einstein delivered three papers that scientists say were, “among the greatest in the history of physics.” One examined the photoelectric effect of Max Planck’s new quantum theory; one was focused on the behavior of small particles in suspension (known as Brownian motion); and one outlined his ‘special theory of relativity’ that made him globally famous. The first paper won Einstein a Nobel Prize and explained the nature of light. The second proved that atoms do exist (a fact that has been in dispute at the time). The third changed the world.
- Einstein: Special Theory of Relativity — Einstein’s special theory of relativity appeared in a paper he wrote called “On the Electrodynamics of Moving Bodies.” Although his famous E=mc2 equation didn’t appear in the original paper, it came later as a supplement. ‘E’ in the equation stands for energy, while ‘m’ is for mass and ‘c2’ is for the speed of light squared. What the equation is saying is that mass and energy are equivalent. They are two forms of the same thing: energy is matter that has already been released, and matter is energy that is waiting to happen. Since ‘c2’ (the speed of light multiplied by itself) is a huge number, the equation is saying that there is a huge amount of energy bound up in every material thing. More on this concept in the below screenshot:

- Einstein: General Theory of Relativity — In 1917, Einstein expanded on his special theory of relativity with his ‘general theory of relativity’, an idea widely recognized as one of the greatest that anyone has ever had. Einstein was bothered by not knowing what happens when a thing in motion — like light — encountered an object like gravity. His general theory of relativity answered that question. But it doesn’t have a huge impact on our everyday lives — it has bigger consequences for things like light, gravity, and the universe itself.
- Quote (P. 125): “In essence what [general] relativity says is that space and time are not absolute, but relative to both the observer and to the thing being observed, and the faster one moves the more pronounced these effects become. We can never accelerate ourselves to the speed of light, and the harder we try (and faster we go) the more distorted we will become, relative to an outside observer.”
- Quote (P. 125): “. . . envision a train one hundred yards long moving at 60 percent of the speed of light. To someone standing on a platform watching it pass, the train would appear to be only eighty yards long and everything on it would be similarly compressed. If we could hear the passengers on the train speak, their voices would sound slurred and sluggish, like a record played at too slow a speed, and their movements would appear similarly ponderous. Even the clocks on the train would seem to be running at only four-fifths of their normal speed. However — and here’s the thing — people on the train would have no sense of these distortions. To them, everything on the train would seem quite normal. It would be we on the platform who looked weirdly compressed and slowed down. It is all to do, you see, with your position relative to the moving object.”
- The Universe Is Expanding — In the 1920s, Edwin Hubble, widely considered the greatest astronomer of the 20th century, discovered that the universe is expanding. Prior to Hubble’s finding, everybody believed the universe was static and stationary. In reality, it is constantly expanding in all directions. A few years earlier, Hubble also proved that there are many galaxies in space. At the time, the belief was that there was one galaxy, the Milly Way, which is our galaxy. Today, astronomers believe there are around 140 billion galaxies in space. The Hubble Space Telescope is named after Hubble.
Ch. 9: The Mighty Atom
- Atoms & Molecules — All things are made of atoms, including air and including us. They are everywhere and constitute everything. And they are unbelievably abundant — they are tiny and are absolutely everywhere. A molecule is simply two or more atoms working together in a stable arrangement: add two atoms of hydrogen to one of oxygen and you will have a molecule of water.
- Quote (P. 133): “The great Caltech physicist Richard Feynman once observed that if you had to reduce scientific history to one important statement it would be ‘All things are made of atoms.’ They are everywhere and they constitute every thing. Look around you. It is all atoms. Not just the solid things like walls and tables and sofas, but the air in between. And they are there in numbers that you really cannot conceive.”
- Atoms Are Recycled — Atoms are incredibly durable and last almost forever. Because of this, atoms are generally recycled through the years. When we die, our atoms go off to become something else. As a result, the atoms that make each of us likely were part of millions of different organisms that existed before us.
- Quote (P. 134): “They are also fantastically durable. Because they are so long lived, atoms really get around. Every atom you possess has almost certainly passed through several stars and been part of millions of organisms on its way to becoming you. We are each so atomically numerous and so vigorously recycled at death that a significant number of our atoms — up to a billion for each of us, it has been suggested — probably once belonged to Shakespeare.”
- Quote (P. 134): “When we die our atoms will disassemble and move off to find new uses elsewhere — as part of a leaf or other human being or drop of dew. Atoms, however, go on practically forever. Nobody actually knows how long an atom can survive, but according to Martin Rees it is probably about 10^35 years — a number so big that even I am happy to express it in notation.”
- Physiology of Atoms — Every atom is made of protons (which have a positive charge), electrons (which have a negative charge), and neutrons (which have no charge). Protons and neutrons are stored in the atom’s nucleus, while electrons swirl on the outside. The nucleus of an atom is tiny but very dense — if the atom itself was a cathedral, the nucleus would be a fly inside the cathedral, but the fly would weigh much, much more than the cathedral. In other words, atoms are mostly empty space. An atom’s chemical identity depends on the number of protons it has. Hydrogen has one proton, for example. Every time a new proton is added, you have a new element. Neutrons don’t influence an atom’s identity, but they do play a big role in the atom’s mass. The number of neutrons usually equals the number of protons, but sometimes there are a few more neutrons than protons. When this happens, you get an isotope. Carbon-14, for example, is an atom of carbon with 6 protons and 8 neutrons.
- Quote (P. 140): “Every atom is made from three kinds of elementary particles: protons, which have a positive electrical charge; electrons, which have a negative electrical charge; and neutrons, which have no charge. Protons and neutrons are packed into the nucleus, while electrons spin around outside. The number of protons is what gives an atom its chemical identity. An atom with one proton is an atom of hydrogen, one with two protons is helium, with three protons is lithium, and so on up the scale. Each time you add a proton you get a new element.”
- Discovery of Neutrons — British physicist James Chadwick discovered the neutron in 1932 while working at Cavendish Laboratory. Chadwick’s discovery came after Ernest Rutherford, a New Zealand-born British physicist, first theorized the existence of neutrons in 1920. Mastery of the neutron was essential to the development of the atomic bomb (Because neutrons have no charge, they aren’t repelled by the electrical fields at the heart of an atom and thus could be fired like tiny torpedoes into an atomic nucleus, setting off the destructive process known as fission).
Ch. 10: Getting the Lead Out
- Lead — Lead is a neurotoxin that can permanently damage the brain if too much of it is consumed. But in the early 1900s, lead was used in a number of consumer products, including gasoline (today, unleaded gasoline is sold because of the health problems lead can cause). Back then, lead was also incredibly profitable to make. So in 1923, General Motors, Du Pont, and Standard Oil of New Jersey teamed up to start Ethyl Gasoline Corporation, which produced a ton of lead. Workers at Ethyl quickly developed health problems working with lead in the factory, and the company was later shut down. Today, lead isn’t found in as many consumer products.
- The Ozone — Ozone is a form of oxygen in which each molecule bears three atoms of oxygen instead of two. Ozone is actually a pollutant at ground level, but it is very beneficial in the stratosphere because it soaks up dangerous ultraviolet radiation that would do a lot of damage to us and our environment if it were not there to protect us. Beneficial ozone is not very abundant, however. That is why it is so easily disturbed and why there is so much debate about chemicals that harm our ozone layer. Chlorofluorocarbons (CFCs) are maybe the most damaging chemicals to our ozone, and they were used in refrigerators and car air conditioners until the 1980s, when it was discovered that they were the main cause of harm to our ozone.
- Reduction In Lead — Scientists in the 1960s began to study snow and ice in Greenland to try to understand how much lead was entering our atmosphere. What they found was that prior to 1923, there was almost no lead in the atmosphere, but a lot of it piled up over time after that year. The Clean Air Act of 1970 was eventually passed, and in 1986 leaded gasoline was not allowed to be sold. This is why, today, the gas we buy is “unleaded.” Almost immediately lead levels in the blood of Americans fell by 80%. But because lead never goes away, people who lived in the 1950s, 60s, 70s, etc. still have a good amount of lead in their blood from breathing it in. Today, lead is still produced via things like mining, smelting, and other industrial activities.
Ch. 11: Muster Mark's Quarks
- The Universe Is Expanding — The universe is actually expanding outward. Objects like galaxies and stars are moving away from Earth faster the farther away they are, at upwards of hundreds of thousands of miles per second — an observation known as Hubble’s Law.
Ch. 12: The Earth Moves
- Plate Tectonics — The Earth’s surface is made up of 8-12 big plates and 20 or so smaller ones that float on the surface of the planet like lily pads. These plates move in different directions and speeds. Some plates are large and fairly inactive, while others are small and move more. These plates are basically segments of Earth that have moved and shifted (very slowly — about 1.5 centimeters per year) over millions and billions of years, contributing to the development of our mountains and other surface level features. Although this is common knowledge today, the idea of plate tectonics wasn’t accepted until 1963, when scientists used magnetic studies of the Atlantic Ocean floor to demonstrate that our sea floors are spreading and our continents are in motion. The boundary of the North American plate traces the outline of America’s western coast (California), which explains why that area experiences so many earthquakes.
- Plate Tectonics: “Conveyer Belts” — Essentially, new ocean crust is formed on both sides of the central rift in the ocean and is pushed away from the rift as new crust comes along behind. The Atlantic floor therefore acts as two conveyer belts, one carrying crust toward North America, and the other carrying crust toward Europe. When crust eventually reaches land, it plunges back into Earth in a process called subduction. This explains where all the sediment goes. It also explains why many ocean floors are dated at 175 million years old when some rocks on our continents are estimated to be billions of years old; ocean floor rocks only last as long as it takes them to travel from the central rift to continental shore.
- Plate Tectonics: What Causes Our Plates to Move? — What causes our plates to move and shift over time? Tremendous heat and pressure within the Earth cause hot magma to flow in what scientists call “convection currents.” These currents are responsible for the sliding of plates. Again, our plates move very slowly — about 1.5 centimeters per year. When you look at the layout of continents around the globe today, you’re only looking at a snapshot. The Earth is shifting as we speak. In another 200 million years, the layout of the continents will look much different — Indonesia and Australia, for example, are actually sinking.
- Quote (P. 182): “Assuming things continue much as at present, the Atlantic Ocean will expand until eventually it is much bigger than the Pacific. Much of California will float off and become a kind of Madagascar of the Pacific. Africa will push northward into Europe, squeezing the Mediterranean out of existence and thrusting up a chain of mountains of Himalayan majesty running from Paris to Calcutta. Australia will colonize the islands to its north and connect by some isthmian umbilicus to Asia. These are future outcomes, but not future events. The events are happening now. As we sit here, continents are adrift, like leaves on a pond. Thanks to Global Positioning Systems we can see that Europe and North America are parting at about the speed a fingernail grows — roughly two yards in a human lifetime. If you were prepared to wait long enough, you could ride from Los Angeles all the way up to San Francisco. It is only the brevity of lifetimes that keeps us from appreciating the changes. Look at a globe and what you are seeing really is a snapshot of the continents as they have been for just one-tenth of 1 percent of the Earth’s history.”
Ch. 13: Bang!
- The Manson Crater — In 1912, a man drilling a well in Manson, Iowa pulled up a lot of strange deformed rock crystalline beneath the surface of the ground there while he was working. The water he was pulling up was also strange — it was very soft water, but naturally occurring soft water had never before been found in Iowa, where the water is very hard and mineralized due to the abundance of limestone in the state. What happened to cause these things? Sometime in the very ancient past, a meteor hit Manson, creating a massive 20-mile-wide, 3-mile-deep crater. Over the next 2.5 million years, ice sheets filled the Manson crater with glacial water, which is why the water was soft in 1912 and the town is as flat as a tabletop. This is considered the biggest event to ever happen in the mainland of the United States.
- Quote (P. 190): “The trauma to Manson’s geology had come not from within the Earth, but from at least 100 million miles beyond. Sometime in the very ancient past, when Manson stood on the edge of a shallow sea, a rock about a mile and a half across, weighing ten billion tons and traveling at perhaps two hundred times the speed of sound ripped through the atmosphere and punched into the Earth with a violence and suddenness that we can scarcely imagine. Where Manson now stands became in an instant a hole three miles deep and more than twenty miles across. The limestone that elsewhere gives Iowa its hard mineralized water was obliterated and replaced by the shocked basement rocks that so puzzled the water driller in 1912.”
- Quote (P. 190): “The Manson impact was the biggest thing that has ever occurred on the mainland United States. Of any type. Ever. The crater it left behind was so colossal that if you stood on one edge you would only just be able to see the other side on a good day. It would make the Grand Canyon look quaint and trifling. Unfortunately for lovers of spectacle, 2.5 million years of passing ice sheets filled the Manson crater right to the top with rich glacial till, then graded it smooth, so that today the landscape at Manson, and for miles around, is as flat as a tabletop.”
- Asteroids — Asteroids are rocky objects orbiting in loose formation between Mars and Jupiter. Nobody knows exactly how many asteroids are tumbling through space, but the number is thought to be more than a billion. Whats freaky about asteroids is that there are so many of them that we have no knowledge of. Asteroids are dark and blend in with space; they only become visible to the naked eye after they heat up as they approach our atmosphere. By then, it’s too late to do anything. In short, asteroids are largely undetectable while they’re in space unless a telescope is trained on them, which is fairly rare.
- Asteroids: Scary Stuff — Think of Earth’s orbit as a freeway on which we are the only car. Millions of pedestrians (asteroids and other space objects) routinely cross our freeway, and about 90% of them are completely unknown to us. In fact, in 1993, an asteroid missed us by just 90,000 miles — the closest passing ever recorded. And we only discovered this asteroid AFTER it had passed us. In other words, there are asteroids and other space objects tumbling by us fairly frequently, and we have no idea beforehand. Scientists believe these asteroid collisions with Earth happen about once every million years. Even a small asteroid could completely crush us and set off a crazy sequence of deadly events. See the screenshots below for a description.
- Quote (P. 194): “Altogether it is thought — though it is really only a guess, based on extrapolating from cratering rates on the Moon — that some two thousand asteroids big enough to imperil civilized existence regularly cross our orbit. But even a small asteroid — the size of a house, say — could destroy a city. The number of these relative tiddlers in Earth-crossing orbits is almost certainly in the hundreds of thousands and possibly in the millions, and they are nearly impossible to track.”


- Asteroids: Dinosaur Extinction — In 1991, a big asteroid collision with Earth some 65 million years ago was named the primary reason for the extinction of dinosaurs. The site of the asteroid impact is a place called the Chicxulub Crater underneath the Yacutan Peninsula in Mexico. Its center is offshore, but the crater is named after the onshore community of Chicxulub Pueblo. The asteroid was about 6 miles in diameter, and the crater is estimated to be 120 miles wide and 12 miles deep. In addition to the crater itself, scientists proved that this event wiped out dinosaurs by finding geological layers with high levels of iridium (a metal common in asteroids). It seems hard to believe that an asteroid could cause so much damage across a planet, but these things are very powerful. In 1994, we were able to witness Comet Shoemaker-Levy 9 collide with Jupiter. The comet dealt serious damage to Jupiter, which silenced the scientists who were skeptical that an asteroid could wipe out a colony of dinosaurs. When the asteroid landed 65 million years ago, it likely caused severe impact damage, wildfires, and a nuclear winter effect that cooled Earth and disrupted the food chain for dinosaurs.
- Quote (P. 202): “The impacts [of Comet Shoemaker-Levy 9] began on July 16, 1994, went on for a week and were bigger by far than anyone — with the possible exception of Gene Shoemaker — expected. One fragment, known as Nucleus G, struck with the force of about six million megatons — seventy-five times more than all the nuclear weaponry in existence. Nucleus G was only about the size of a small mountain, but it created wounds in the Jovian surface the size of Earth.”
- Interesting Fact — There are 15 million hogs in Iowa!
Ch. 14: The Fire Below
- Earth’s Interior — The distance from the surface of the Earth to its center is 3,959 miles, which isn’t all that far. The world beneath us is composed of four layers — rocky outer crust, a mantle of hot rock, a liquid outer core, and a solid inner core. Our attempts to penetrate Earth have been feeble — most mines on Earth go no more than a quarter mile beneath the surface. If the planet were an apple, we wouldn’t have broken through the skin. Nobody knows how hot the Earth’s core is, but estimates range from 7,000-13,000 degrees Fahrenheit.
- Earthquakes — The most common types of earthquakes are those where two plates meet. The San Andreas Fault in California is a good example. As the plates push up against each other, pressure builds up until one or the other gives way, causing an earthquake. Earthquakes are fairly common; every day, on average, two earthquakes of magnitude 2.0 or greater go off somewhere on the world. The largest earthquake since the Richter scale’s invention was either one centered on Prince William Sound in Alaska in March 1964, which measured 9.2 on the Richter scale, or one in the Pacific Ocean off the coast of Chile in 1960, which was initially logged at 8.6 magnitude but later revised to a whopping 9.5.
- Earthquakes: Danger in Tokyo — Tokyo sits on the boundary of three tectonic plates in a country that has already experienced significant seismic activity. It’s a city that some experts have described as “the city waiting to die.” In 1995, the city of Kobe, about 300 miles west of Tokyo, experienced a 7.2 quake that killed more than 6,000 people. And in 1923, Tokyo itself suffered one of the most devastating quakes of the modern era. The earthquake is known as the Great Kano quake — an event that was 10 times more powerful than the Kobe earthquake and killed more than 200,000 people. Tokyo has been eerily quiet since then, so the strain beneath the surface has been building for more than 100 years now.
- Mount St. Helens Eruption — On May 18, 1980, Mount St. Helens in Washington erupted, sparking the biggest landslide in human history. It had been spewing small amounts of magma for a few months before finally bursting. Interestingly, it erupted laterally (rather than vertically), which created a lot more damage.
- Quote (P. 221): “At 8:32 AM on a Sunday morning, May 18, the north side of the volcano collapsed, sending an enormous avalanche of dirt and rock rushing down the mountain slope at 150 miles an hour. It was the biggest landslide in human history and carried enough material to bury the whole of Manhattan to a depth of four hundred feet. A minute later, its flank severely weakened, St. Helens exploded with the force of five hundred Hiroshima-sized atomic bombs, shooting out a murderous hot cloud at up to 650 miles an hour — much too fast, clearly, for anyone nearby to outrace. Many people who were thought to be in safe areas, often far out of sight of the volcano, were overtaken. Fifty-seven people were killed. Twenty-three of the bodies were never found.”
- Quote (P. 222): “Mount St. Helens lost thirteen hundred feet of peak, and 230 square miles of forest were devastated. Enough trees to build 150,000 homes (or 300,000 in some reports) were blown away. The damage was placed at $2.7 billion. A giant column of smoke and ash rose to a height of sixty thousand feet in less than ten minutes. An airliner some thirty miles away reported being pelted with rocks. Ninety minutes after the blast, ash began to rain down on Yakima, Washington, a community of fifty thousand people about eighty miles away. As you would expect, the ash turned day to night and got into everything, clogging motors, generators, and electrical switching equipment choking pedestrians, blocking filtration systems, and generally bringing things to a halt. The airport shut down and highways in and out of the city were closed.”
Ch. 15: Dangerous Beauty
- Yellowstone: A Disaster Waiting to Happen — Yellowstone National Park features 2.2 million acres of wilderness recreation area atop a gigantic active volcanic hot spot. Mostly in Wyoming, the park spreads into parts of Montana and Idaho as well. Yellowstone is the largest active volcano on Earth. At some point in the past, Yellowstone blew up with a violence unknown to man. In its wake, it left a giant, flat volcanic caldera/hot spot pit, which basically encompasses the entire park. Visitors of the park are basically shuffling around on a 40+-mile long hot spot. When this thing erupts, it’s going to be wild. Yellowstone first erupted 16 million years ago and has blown up hundreds up times since. The last time it blew up, the blast was 1,000 times greater than Mount St. Helens and provided enough ash to completely bury New York City. On average, Yellowstone has erupted every 600,000 years, but it’s been 630,000 years since its last eruption. It’s due.
- Quote (P. 225): “Yellowstone, it turns out, is a supervolcano. It sits on top of an enormous hot spot, a reservoir of molten rock that rises from at least 125 miles down in the Earth. The heat from the hot spot is what powers all of Yellowstone’s vents, geysers, hot springs, and popping mud pots. Beneath the surface is a magma chamber that is about forty-five miles across — roughly the same dimensions as the park — and about eight miles thick at its thickest point. Imagine a pile of TNT about the size of Rhode Island and reaching eight miles into the sky, to about the height of the highest cirrus clouds, and you have some idea of what visitors to Yellowstone are shuffling around on top of.”
- Not All Mountains Are Volcanoes — Many mountains are volcanoes, but not all of them are. Some mountains have no magma inside them; they were formed when two tectonic plates rammed into each other. But many mountains are volcanic and feature cones at the top that are formed when erupting magma accumulates in a symmetrical mound. This “cone” is what makes these volcanoes give off the appearance of mountains. In short, for a mountain to be a volcano and a volcano to be a mountain, it must be formed from magma and volcanic materials from below the Earth’s surface. And some volcanoes are simply giant hot spot pits and don’t resemble a mountain at all (e.g. Yellowstone).
- Yellowstone: A Risky Place — As beautiful as it is, Yellowstone is a very risky place to visit. While there, you’re moving around atop the largest active volcano in the world, and one that is overdue for an explosion. The park has more geysers than any place on the planet, and new ones are popping up all the time. These geysers can pop up unannounced literally anywhere in the park. Additionally, many of the pools of water in the park are dangerously hot. Rock slides are fairly routine as well. There are also many earthquakes since the park sits near a fault line. It’s a fascinating pace to visit, but one that is also a little uncomfortable.
Ch. 16: Lonely Planet
- Water Outweighs Air — Water is about 1,300 times heavier than air, which is why our bodies crumble under the pressure of deep ocean exploration. On land, if you ascend 500 feet to climb something, you wouldn’t feel much different from the change in pressure. But underwater, the same 500-foot dive would cause the veins and lungs in your body to compress. Why? Because we are made mostly of water (water in our cells), it’s the gases in our body that cause the compression and collapse of our internal organs. Roughly 80% of the air we breathe is nitrogen — when exposed to pressure changes underwater, the nitrogen gas in our body dissolves into our tissues and leads to problems. Deep divers have proven that we can handle such changes in pressure underwater for a very short time, but prolonged exposure to that kind of pressure will lead to serious problems.
- Quote (P. 239): “On land, if you rose to the top of a five-hundred-foot eminence — Cologne Cathedral or the Washington Monument, say — the change in pressure would be so slight as to be indiscernible. At the same depth underwater, however, your veins would collapse and your lungs would compress to the approximate dimensions of a Coke can.”
- Quote (P. 240): “Most oceans are of course much shallower, but even at the average ocean depth of two and a half miles the pressure is equivalent to being squashed beneath a stack of fourteen loaded cement trucks.”
- Quote (P. 241): “The air we breathe is 80 percent nitrogen. Put the human body under pressure, and that nitrogen is transformed into tiny bubbles that migrate into the blood and tissues. If the pressure is changed too rapidly — as with a too-quick ascent by a diver — the bubbles trapped within the body will begin to fiz in exactly the manner of a freshly opened bottle of champagne, clogging tiny blood vessels, depriving cells of oxygen, and causing pain so excruciating that sufferers are prone to bend double in agony — hence ‘the bends.’”
- Living On Earth: Lucky Breaks — With everything that goes on in our universe and galaxies, we are very fortunate to live on a planet like Earth that is actually habitable. And even on Earth, we only have the ability to live on about 12% of the land on the planet. That number falls to 4% when looking at land and sea. In other words, we are very lucky. A small percentage of Earth is livable for us, but we have found ourselves here anyway. There are four core breaks that went our way to make Earth habitable for us:
- Excellent Location — We are a perfect distance from the sun, and the sun is the perfect size. If the sun was any larger, it would have burned out already. If our orbit around the sun was any closer or further, we would be dead. Any closer and the sun’s energy would melt is. Venus is a good example of this — it is only 25 million miles closer to the sun than us and the sun’s rays reach Venus only two minutes earlier than they reach us, yet Venus’s surface temperature is 900 degrees Fahrenheit. And if our orbit was any further, we’d be frozen to death. The icy planet of Mars is a good example.
- Molten Interior — Earth’s molten interior, including the magma swirling around beneath us, created the atmosphere that magnetic fields that protect us and gave us plate tectonics. If Earth were perfectly smooth, with no plates, we would be all ocean. Our interior is responsible for the land we have on our planet.
- Quote (P. 248): “I don’t imagine even many geophysicists, when asked to count their blessings, would include living on a planet with a molten interior, but it’s a pretty near certainty that without all that magma swirling around beneath us we wouldn’t be here now. Apart from much else, our lively interior created the outgassing that helped to build an atmosphere and provided us with the magnetic field that shields us from cosmic radiation. It also gave us plate tectonics, which continually renews and rumples the surface. If Earth were perfectly smooth, it would be covered everywhere with water to a depth of four kilometers. There might be life in that lonesome ocean, but there certainly wouldn’t be baseball.”
- The Moon — Compared to other moons in the universe, our Moon is really big. The Moon’s gravitational pull keeps us spinning at the right speed and angle necessary to sustain stable life. Interestingly, the Moon is slipping away from us at a rate of about 1.5 inches per year. In a few million years, it may pull away from us altogether, and we will lose the stability it provides us. Again, our Moon is believed to have been created after a Mars-sized object slammed into Earth 4.5 billion years ago, blowing out enough material to create a Moon from the debris.
- Quote (P. 249): “Without the Moon’s steadying influence, the Earth would wobble like a dying top, with goodness knows what consequences for climate and weather. The Moon’s steady gravitational influence keeps the Earth spinning at the right speed and angle to provide the sort of stability necessary for the long and successful development of life. This won’t go on forever. The Moon is slipping from our grasp at a rate of about 1.5 inches a year. In another two billion years it will have receded so far that it won’t keep us steady and we will have to come up with some other solution, but in the meantime you should think of it as much more than just a pleasant feature in the night sky.”
- Timing — The sequence of events, triumphs, and challenges that have taken place over the last 4.6 billion years have led us to where we are today. Without everything playing out as it has, we may not be here at all.
- Elements on Earth — There are 92 naturally-occurring elements on Earth, plus another twenty or so that have been created in labs. Oxygen is the most abundant element, accounting for just under 50% of the Earth’s crust. Silicon is the second most common element on Earth. Carbon is the 15th most common element (0.048% of Earth’s crust), yet we would be doomed without it. Carbon latches onto many other atoms (including itself) and holds tightly, helping to form molecules, DNA, and proteins. In terms of inside the body, hydrogen is the most abundant molecule, followed by oxygen and carbon — for every 200 atoms in the body, 126 are hydrogen, 51 are oxygen, and 19 are carbon.
Ch. 17: Into the Troposphere
- The Atmosphere: Our Protector — Our atmosphere protects us from cosmic rays, ultraviolet rays from the sun, and other charged particles swirling around in the universe. It also keeps us warm. Interestingly, our atmosphere only extends upward to 120 miles. Our atmosphere is technically made up of four layers: troposphere, stratosphere, mesosphere, and ionosphere (now called the thermosphere). The troposphere is probably the most important to us as it alone contains enough warmth and oxygen to allow us to function.
- Quote (P. 255): “Thank goodness for the atmosphere. It keeps us warm. Without it, Earth would be a lifeless ball of ice with an average temperature of minus 60 degrees Fahrenheit. In addition, the atmosphere absorbs or deflects incoming swarms of cosmic rays, charged particles, ultraviolet rays, and the like. Altogether, the gaseous padding of the atmosphere is equivalent to a fifteen-foot thickness of protective concrete, and without it these invisible visitors from space would slice through us like tiny daggers. Even raindrops would pound us senseless if it weren’t for the atmosphere’s slowing drag.”
- The Troposphere — The troposphere is about 10 miles thick at the equator and 6-7 miles thick in the temperate latitudes where we live. About 80% of the atmosphere’s mass, almost all of its water, and therefore nearly all of its weather are all contained within this thin layer that we call the troposphere.
- Temperatures Above — The temperature of the air in certain layers of the atmosphere can vary wildly. The temperature six miles up can be -70 degrees, and you would need supplementary oxygen. After you leave the troposphere, the temperature warms up to 40 degrees thanks to the ozone. It then plunges to -130 degrees in the mesosphere before jumping to 2,700 degrees or more in the thermosphere. In the thermosphere, the temperature can go up and down by thousands of degrees routinely. Together, these layers somehow manage to create a livable temperature for us here at ground level.
- How Temperature Works — Temperature is really just a function of the activity of molecules in the air. At sea level, air molecules are so thick that molecules are jam-packed together and can’t move very far without crashing into another molecule. This friction between trillions of molecules is what creates heat and higher air temperatures. But at really high elevations in the atmosphere, there are fewer molecules around, which means there is more space between them. Because there is more space, there isn’t as much friction and banging around between molecules, meaning less heat is exchanged, leading to colder air temperatures. This explains why the temperature drops about 3 degrees with every thousand feet you climb. It also explains why space is so cold. And the sun aids this process by providing energy to the atoms, causing them to bounce around more. This is why it gets cooler overnight; the sun is down and there is less energy being supplied to the molecules.
- Quote (P. 256): “Temperature is really just a measure of the activity of molecules. At sea level, air molecules are so thick that one molecule can move only the tiniest distance — about three-millionths of an inch to be precise — before banging into another. Because trillions of molecules are constantly colliding, a lot of heat gets exchanged. But at the height of the thermosphere, at fifty miles or more, the air is so thin that any two molecules will be miles apart and hardly ever come in contact. So although each molecule is very warm, there are few interactions between them and thus little heat transference. This is good news for satellites and spaceships because if the exchange of heat were more efficient any man-made object orbiting at that level would burst into flame.”
- Quote (P. 258): “The answer again takes us back to the question of the density of molecules in the atmosphere. Sunlight energizes atoms. It increases the rate at which they jiggle and jounce, and in their enlivened state they crash into one another, releasing heat. When you feel the sun warm on your back on a summer’s day, it’s really excited atoms you feel. The higher you climb, the fewer molecules there are, and so the fewer collisions between them.”
- Air Is Heavy — We usually think of air as being weightless, but it most certainly is not. There are about 5,200 million million tons of air around us — a huge amount of air. When you get a lot of wind whipping that air around, it’s no surprise that crazy things like thunderstorms, hurricanes, and tornados can happen. At any one moment, 1,800 thunderstorms are in progress around the globe — about 40,000 per day. Day and night across the planet, about 100 lightning bolts hit the ground every second. The sky is a wild place.
- Quote (P. 259): “But get air in motion, as with a hurricane or even a stiff breeze, and you will quickly be reminded that it has very considerable mass. Altogether there are about 5,200 million million tons of air around us — 25 million tons for every square mile of the planet — a not inconsequential volume. When you get millions of tons of atmosphere rushing past at thirty or forty miles an hour, it’s hardly a surprise that limbs snap and roof tiles go flying. As Anthony Smith notes, a typical weather front may consist of 750 million tons of cold air pinned beneath a billion tons of warmer air. Hardly a wonder that the result is at times meteorologically exciting.”
- Convection & Air Pressure — The process that moves air around in the atmosphere is the same process that drives the internal engine of the planet, namely convection. Moist, warm air from the equatorial regions rises until it hits the barrier of the tropopause and spreads out. As it travels away from the equator and cools, it sinks. When it hits bottom, some of the sinking air looks for an area of low pressure to fill and heads back for the equator, completing the circuit. At the equator the convection process is generally stable and the weather predictably fair, but in temperate zones the patterns are far more seasonal, localized, and random, which results in an endless battle between systems of high-pressure air and low. Low-pressure systems are created by rising air, which conveys water molecules into the sky, forming clouds and eventually rain. Warm air can hold more moisture than cool air, which is why tropical and summer storms tend to be the heaviest. Thus low areas tend to be associated with clouds and rain, and highs generally spell sunshine and fair weather.
- Wind: Why It Happens — The Sun unevenly distributed heat. As a result, the planet is filled with pockets of uneven air pressure. Air doesn’t like this, so it swirls around frantically to try to even everything out and create equilibrium in air pressure across the planet. In essence, wind is just the air’s way of trying to create balance. Air always flows from areas of high pressure to areas of low pressure — so the greater the difference between the two, the harder the wind blows in an effort to restore balance.
- Quote (P. 261): “What we do know is that because heat from the Sun is unevenly distributed, differences in air pressure arise on the planet. Air can’t abide this, so it rushes around trying to equalize things everywhere. Wind is simply the air’s way of trying to keep things in balance. Air always flows from areas of high pressure to areas of low pressure (as you would expect; think of anything with air under pressure — a balloon or an air tank — and think how insistently that pressured air wants to get someplace else), and the greater the discrepancy in pressures the faster the wind blows.”
- Interesting Fact — Thermometers were difficult to put together at first. Two of the first people to do it successfully were Daniel Gabriel Fahrenheit and Anders Celsius. Fahrenheit built the world’s first accurate thermometer in 1717, putting freezing at 32 degrees and boiling at 212 degrees. The weird degree scale bothered people, so Celsius built another thermometer in 1742 that put freezing at 0 degrees and boiling at 100. These scales are named after Fahrenheit and Celsius, respectively.
- Rainfall — Clouds contain a small amount of rain inside them, less than what most people think. When it does rain and clouds empty rain drops, the fate of every raindrop really depends on where it lands. If it lands in fertile soil it will be soaked up by plants or reevaporated directly within hours or days. If it finds its way down to the groundwater, however, it may not see sunlight again for many years — thousands if it gets really deep. When you look at a lake, you are looking at a collection of molecules that have been there on average for about a decade. In the ocean the residence time is thought to be more like a hundred years. Altogether about 60% of water molecules in a rainfall are returned to the atmosphere within a day or two. Once evaporated, they spend no more than a week or so before falling again as rain.
- Carbon Dioxide Overload — Humans put up about 360 parts per million of carbon into the air every year via factories and car emissions. Nature, mostly via volcano activity and the decay of trees and plants, puts up another 200 billion tons of carbon dioxide into the atmosphere. That’s a lot of carbon. Our oceans (specifically crustaceans and other tiny organisms underneath the surface), rocks, and forests are what lock away a lot of the carbon and keep us stable. The fear around too much carbon is that Earth would experience a runaway increase in its warming. Trees and forests would die as a result and release even more stored carbon into the atmosphere, creating additional warming.
Ch. 18: Into the Troposphere
- Issues With Salt — We need a little bit of salt to survive, but we cannot handle salt water. Our oceans are loaded with salt — a typical liter of seawater contains about 2.5 teaspoons of common salt — far more than our bodies have the ability to handle. When we get too much salt in our system, our cells dispatch water from inside them in an effort to dilute the salt in the body. When this happens, we get dehydrated and bad things start to happen.
- Quote (P. 272): “Take a lot of salt into your body and your metabolism very quickly goes into crisis. From every cell, water molecules rush off like so many volunteer firemen to try to dilute and carry off the sudden intake of salt. This leaves the cells dangerously short of the water they need to carry out their normal functions. They become, in a word, dehydrated. In extreme situations, dehydration will lead to seizures, unconsciousness, and brain damage. Meanwhile, the overworked blood cells carry the salt to the kidneys, which eventually become overwhelmed and shut down. Without functioning kidneys you die. That is why we don’t drink seawater.”
- Water Is Old — There are 320 million cubic miles of water on Earth and that is all we’re ever going to get. The system is closed: nothing can be added or subtracted. Water is constantly recycled through a natural process called the water cycle that has been occurring for billions of years. The water you drink has been around doing its job since the Earth was young. By 3.8 billion years ago, the oceans had (at least more or less) achieved their present volumes.
- Water Dominates — About 97% of all the water on Earth is in the oceans, the greater part of it in the Pacific, which covers half the planet and is bigger than all the landmasses put together. Altogether the Pacific holds just over half of all the ocean water (51.6%); the Atlantic has 23.6% and the Indian Ocean 21.2%, leaving just 3.6% to be accounted for by all the other seas. A potato is 80% water, a cow 74%, a bacterium 75%. A tomato, at 95%, is almost all water. Even humans are 65% water, making us more liquid than solid by a margin of almost two to one.
- Alvin — Alvin is a mini submarine built to help us investigate life under the surface of our oceans. The truth is, we don’t know much about what goes on in our oceans — in fact, we have better maps of Mars than our own seabeds. Alvin was built in the 1960s by (remarkably) General Mills, the food company, and remains America’s premier research vessel. There are only a handful of vessels that can reach the depths of the “abyssal plain” — the deep ocean floor that covers more than half of the planet’s surface — and Alvin is one of them.
- Great Blue Whales & Giant Squids— Great blue whales are the biggest creatures to ever appear on Earth, bigger than dinosaurs. Their tongue weigh as much as an elephant, their heart is a big as a car, and some of their blood vessels are wide enough to swim through. Giant squid are also huge animals, though no scientist has ever seen one alive — when they are found, they are usually dead and wash up on beaches. That said, they must all over the place because they are a central part of a whale’s diet. Interesting fact about this below:
- Quote (P. 282): “The indigestible parts of giant squid, in particular their beaks, accumulate in sperm whales’ stomachs into the substance known as ambergris, which is used as a fixative in perfumes. The next time you spray on Chanel No. 5 (assuming you do), you may wish to reflect that you are dousing yourself in distillate of unseen sea monster.”
Ch. 19: The Rise of Life
- Proteins & Amino Acids — Proteins are the workhorses of the biological world, and have an impact on so many reactions in the body. Proteins are produced when amino acids are strung together in an exact sequence. Different amino acid chains code different proteins, and there are possible more than a million types of proteins in the human body. When you think about it, it’s pretty miraculous — amino acids need to be strung in a perfect sequence to form a certain protein, and one mishap will screw it all up. Some proteins have really long chains, while others are shorter (e.g. the hemoglobin protein is just 146 amino acids long, while collagen is 1,055 amino acids long).
- Quote (P. 288): “Proteins are what you get when you string amino acids together, and we need a lot of them. No one really knows, but there may be as many as a million types of protein in the human body, and each one is a little miracle. By all the laws of probability proteins shouldn’t exist. To make a protein you need to assemble amino acids in a particular order, in much the same way that you assemble letters in a particular order to spell a word. The problem is that words in the amino acid alphabet are often exceedingly long. To spell collagen, the name of a common type of protein, you need to arrange eight letters in the right order. But to make collagen, you need to arrange 1,055 amino acids in precisely the right sequence.”
- Proteins, DNA, and Cells — Once proteins are built, they need to fold themselves up and replicate. But they can’t replicate on their own — they need DNA to help with that. DNA is excellent at replicating code and reproducing proteins. After that, proteins and DNA need a little compartment to operate in, otherwise they would be cool but useless chemicals in the body. This is where cells come in. By providing a base for DNA, proteins, and other very important components of life, cells literally bring us to life.
- Quote (P. 289): “A protein to be of use must not only assemble amino acids in the right sequence, but then must engage in a kind of chemical origami and fold itself into a very specific shape. Even having achieved this structural complexity, a protein is no good to you if it can’t reproduce itself, and proteins can’t. For this you need DNA. DNA is a whiz at replicating — it can make a copy of itself in seconds — but can do virtually nothing else. So we have a paradoxical situation. Proteins can’t exist without DNA, and DNA has no purpose without proteins.”
- Quote (P. 289): “And there is more still. DNA, proteins, and the other components of life couldn’t prosper without some sort of membrane to contain them. No atom or molecule has ever achieved life independently. Pluck any atom from your body, and it is no more alive than is a grain of sand. It is only when they come together within the nurturing refuge of a cell that these diverse materials can take part in the amazing dance that we call life. Without the cell, they are nothing more than interesting chemicals. . . It is little wonder that we call it the miracle of life.”
- Evolution of Life on Earth — Earth was created about 4.5 billion years ago with the Big Bang. Life on Earth began about 3.5-3.7 billion years ago. The first life forms on Earth were tiny bacteria that all operated in our oceans because there was not enough oxygen to breathe outside of the seas. In other words, life on Earth began in the water. This is because there was less oxygen to breathe on Earth back then than there is on Mars today. Below is a brief summary of how early life on Earth evolved from water to land.
- Bacteria — The very first life forms on Earth were tiny bacterial organisms in the ocean. In fact, for two billion years, all we were was bacteria.
- Cyanobacteria — At some point in the first billion years of life, cyanobacteria, which were blue-green algae, learned to use the huge amount of hydrogen available in water to absorb water molecules (therefore taking in hydrogen) and release oxygen as waste. By doing this, cyanobacteria invented photosynthesis, which is arguably the most important metabolic innovation in the history of life because it led to the widespread production of oxygen on Earth. As more and more cyanobacteria came to be, they released more and more oxygen.
- Oxygen Organisms — The production of oxygen led to the growth of oxygen-using organisms like cyanobacteria. Most other organisms that did not adapt to oxygen died off.
- Stromatolites — Millions of years later, stromatolites began to emerge in shallow water. These were cyanobacteria that had evolved to become sticky. Because they were stickier, dust and sand attached to them, which made them more solid in structure. Sometimes stromatolites looked like cauliflower, sometimes they looked like mattresses. Other times, they took other forms. But all of them were basically living rocks that existed at the surface of water, or just underneath. Stromatolites continued to produce oxygen, and over the next two billion years, these rocks raised the level of oxygen on Earth to 20%, setting the table for the next life forms on land. Interestingly, there are living stromatolites on exhibit at Shark Bay in Australia. Worth visiting.
- Single Cell Organisms — Eventually, oxygen levels in our atmosphere finally reached levels that we experience today. When this happened, organelles called prokaryotes and eukaryotes appeared on land. Eukaryotes were single-celled organisms with a nucleus, mitochondria, and other important structures, and they took us from a world full of only bacteria to a world with nucleus-bearing organisms. Gradually, a system evolved in which life was dominated by two types: organisms that expel oxygen (e.g. plants) and those that take it in (e.g. humans and animals). Humans and animals are eukaryotes. Bacteria are single-cells organisms that don’t have a nucleus.
- Multicellular Beings — At first, eukaryotes were single-celled organisms that didn’t do much of anything other than exist. A billion years went by before they finally mastered a really great trick: They learned to form together into multicellular beings. Thanks to this innovation, big, complicated, visible organisms like us were made possible.
Ch. 20: Small World
- A Bacterial World — Don’t get it twisted; this is a world of bacteria and microbes, and we’re able to be here simply because they let us. There is bacteria literally everywhere. We have trillions of bacteria cells on our skin and inside our body. In fact, by most accounts, the bacteria cells we have on and within us outnumber our human cell count by a ratio of 10:1. As touched on in the previous chapter, life on Earth began with bacteria and microbes. They were here far before us, and they will be here until the Earth collapses.
- Quote (P. 302): “In fact, there is no point in trying to hide from your bacteria, for they are on and around you always, in numbers you can’t conceive. If you are in good health and averagely diligent about hygiene, you will have a herd of about one trillion bacteria grazing on your fleshy plains — about a hundred thousand of them on every square centimeter of skin. They are there to dine off the ten billion or so flakes of skin you shed every day, plus all the tasty oils and fortifying minerals that seep out from every pore and fissure. You are for them the ultimate food court, with the convenience of warmth and constant mobility thrown in. . . And those are just the bacteria that inhabit your skin. There are trillions more tucked away in your gut and nasal passages, clinging to your hair and eyelashes, swimming over the surface of your eyes, drilling through the enamel of your teeth. Your digestive system alone is host to more than a hundred trillion microbes, of at least four hundred types.”
- Quote (P. 303): “Every human body consists of about 10 quadrillion cells, but about 100 quadrillion bacterial cells [10:1 ratio]. They are, in short, a big part of us. From the bacteria’s point of view, of course, we are a rather small part of them.”
- Quote (P. 303): “Bacteria may not build cities or have interesting social lives, but they will be here when the Sun explodes. This is their planet, and we are on it only because they allow us to be.”
- Quote (P. 311): “Indeed, according to [Carl] Woese, if you totaled up all the biomass of the planet — every living thing, plants included — microbes would account for at least 80 percent of all there is, perhaps more. The world belongs to the very small — and it has for a very long time.”
- Bacteria: Helping Us Out — Fortunately, many of the bacteria cells that live on and within us do some great things for us. They can be thought of as “good bacteria.” In fact, we wouldn’t be able to live without some of the functions good bacteria carry out. They process waste and help us digest food; they make our water and soil clean; they help us synthesize vitamins; and they help attack invaders in our body. They also supply the majority of the oxygen we breathe.
- Quote (P. 303): “Bacteria, never forget, got along for billions of years without us. We couldn’t survive a day without them. They process our wastes and make them usable again; without their diligent munching nothing would rot. They purify our water and keep our soils productive. Bacteria synthesize vitamins in our gut, convert the things we eat into useful sugars and polysaccharides, and go to war on alien microbes that slip down our gullet. We depend totally on bacteria to pluck nitrogen from the air and convert it into useful nucleotides and amino acids for us. It is a prodigious and gratifying feat. . . Microbes, including the modern versions of cyanobacteria, supply the greater part of the planet’s breathable oxygen.”
- Bacteria: Replication & DNA Swapping — Bacteria cells are prolific at multiplying and swapping information. They can generate 280 billion individuals in a single day, whereas a human cell can divide about once in a day. Bacteria also share information; they can literally take pieces of DNA and genetic coding from each other. It’s not possible in humans, but it would be like two friends swapping genes to get different eye colors. What this means (from a genetic point view) is that bacteria is kind of like a super-organism that is spread out into trillions of tiny, dispersed pieces — it can adapt very quickly because of its ability to swap information. This is partly why bacteria is practically indestructible and has been known to exist literally everywhere and in any conditions: Bacteria has been found living in boiling mud pots, deep inside rocks, at the bottom of the ocean, in pools of icy water, and even in a sealed camera lens that sat on the Moon for two years.
- Bad Bacteria — It’s worth remembering that most bacteria are either neutral or good bacteria. That said, bad bacteria (also called microbes and pathogens) can cause major problems and was the No. 1 killer in the days before antibiotics. Why would bad bacteria want to harm its host? For one, causing the host to be sick can make it easier for the microbe to spread. Vomiting and sneezing allows the microbe to spread to others easily. Microbes also like to spread by enlisting a third party (e.g. a mosquito) to get into the bloodstream of other organisms. This is why many really bad diseases (e.g. malaria, yellow fever, and hundreds of others) began with a mosquito bite. In the end, the only time bad bacteria cares about you is when it kills you too fast, because that means the bacteria will likely also die.
- Quote (P. 312): “Making a host unwell has certain benefits for the microbe. The symptoms of an illness often help to spread the disease. Vomiting, sneezing, and diarrhea are excellent methods of getting out of one host and into position for another. The most effective strategy of all is to enlist the help of a mobile third party. Infectious organisms love mosquitoes because the mosquito’s sting delivers them directly to a bloodstream where they can get straight to work before the victim’s defense mechanisms can figure out what’s hit them. This is why so many grade-A diseases — malaria, yellow fever, dengue fever, encephalitis, and a hundred or so other less celebrated but often rapacious maladies — begin with a mosquito bite.”
- Why We Feel Sick — When we’re sick, most of the symptoms we feel are a result of our body fighting the bad microbes. Rather than the pathogen itself, what you feel is the war going on inside you as your white blood cells spring into action and begin hunting the pathogen. While the war is being waged, certain cells and tissues in the body can be damaged, leading to symptoms of sickness. There is more good information on white blood cells and our immune response in The Body, also by Bill Bryson.
- Quote (P. 313): “A great deal of sickness arises not because of what the organism has done to you but what your body is trying to do to the organism. In its quest to rid the body of pathogens, the immune system sometimes destroys cells or damages critical tissues, so often when you are unwell what you are feeling is not the pathogens but your own immune responses.”
- Bacteria Out of Place — Sometimes bacteria gets in the wrong place in the body. When this happens, chaos can ensue. In this way, some forms of bacteria seem to be specifically designed for certain spots in the body. One of the ways this can happen is during a major physical event, like a car crash. In situations such as these, microbes from the gut, for example, can get jolted into the bloodstream or other parts of the body and cause major infections. Meningitis is an example of this. At least 10% of adults, and 30% of teenagers, carry this deadly bacteria, but it normally operates harmlessly in the throat. But very occasionally, it gets into a person’s bloodstream and it makes them very ill, many times killing them. To protect against this bacteria, many college-bound kids get a meningitis shot.
- Quote (P. 314): “One of the odder aspects of infection is that microbes that normally do no harm at all sometimes get into the wrong parts of the body and ‘go kind of crazy’, in the words of Dr. Bryan Marsh, an infectious diseases specialist at Dartmouth-Hitchcock Medical Center in Lebanon, New Hamphire. ‘It happens all the time with car accidents when people suffer internal injuries. Microbes that are normally benign in the gut get into other parts of the body — the bloodstream, for instance — and cause terrible havoc.’”
- Antibiotics: Microbes Are Adapting — As noted earlier, bacteria are very good at swapping information and genetic code, which allows them to adapt to conditions easily. This is a problem when it comes to antibiotics. By aiding our body in the fight against infection, antibiotics have saved countless lives since they were first introduced. But one of the big concerns here is that bacteria are beginning to resist the series of antibiotics we’ve discovered. The more we expose microbes to antibiotics, the more opportunity they have to develop resistance to the medicine. In 1945, a typical case of pneumonia could be knocked out with 40,000 units of penicillin. Today, because of increased resistance, it can take more than 20 million units per day to achieve the same result. On some diseases, penicillin is no longer effective. What’s worse is that antibiotics are often prescribed when they aren’t needed and farm animals are given antibiotics to fatten them, meaning Americans consume secondhand antibiotics through their food. All of this adds to antibiotic resistance.
- Viruses vs. Bacteria — Unlike bacteria, viruses are not living things. In fact, they are completely harmless things independently. It’s when they are inserted into a host that they can cause real damage. When they enter a host, viruses come alive by hijacking one of the host’s cells, turning it into a rogue, infected cell, and then multiplying over and over. About 5,000 viruses are known, and they include things like the common flu, common cold, smallpox, rabies, Ebola, polio, and HIV/AIDS. Because they are not living things, viruses are usually very simple and only have a few genes. When they are not inside of a host, they usually only live for a few hours.
- Quote (P. 316): “Smaller and simpler than bacteria, viruses aren’t themselves alive. In isolation they are inert and harmless. But introduce them into a suitable host and they burst into busyness — into life. About five thousand types of virus are known, and between them they afflict us with many hundreds of diseases, ranging from the flu and common cold to those that are most invidious to human well-being: smallpox, rabies, yellow fever, ebola, polio, and the human immunodeficiency virus, the source of AlDS.”
- Quote (P. 316): “Viruses prosper by hijacking the genetic material of a living cell and using it to produce more virus. They reproduce in a fanatical manner, then burst out in search of more cells to invade.”
- Viruses: The Spanish Flu — One of the deadliest viruses of all time (including COVID) was the Spanish Flu of 1918, also known as the Great Swine Flu, which killed 21 million people in its first four months. World War I killed that many people in four years. Similar to COVID, many scientists believe it spread across the globe so quickly because many people who had the virus did not experience symptoms. This allowed the virus to spread very fast. At least 50 million people ended up dying from this virus. For context, about 4.7 million died from COVID globally.
Ch. 21: Life Goes On
- Life Is Resilient — When you look back at the history Earth and everything that has happened on this planet, it’s pretty impressive to think that life has survived ice ages, asteroid hits, volcanic eruptions, and other major events. Life on Earth is very resilient. Throughout everything that has happened, life on Earth has adapted and pushed forward.
Ch. 22: Good-Bye to All That
- Life On Earth: A Timeline — To help readers get an understanding of when things happened and how recent humans are, Bryson begins this chapter with a brief timeline of life on Earth using a 24-hour clock. Earth began at midnight with the Big Bang (4.5 billion years ago). The rest of the timeline is as follows:
- 4 AM — The first single-celled organisms (bacteria/microbes) appear. Nothing more happens for the next 16 hours.
- 8:30 PM — At 8:30 PM, all we have on Earth is a bunch of bacteria/microbes.
- 8:40 PM — Sea plants appear
- 9 PM — The first jellyfish appear
- 9:55 PM — Plants begin to appear on land (450 million years ago)
- 10:15 PM — The first land creatures appear (400 million years ago). These may have looked like oxygen-breathing fish, something like a modern mudskipper.
- Quote (P. 338): “Popular illustrations have encouraged us to envision the first venturesome land dwellers as a kind of ambitious fish — something like the modern mudskipper, which can hop from puddle to puddle during droughts — or even as a fully formed amphibian. In fact, the first visible mobile residents on dry land were probably much more like modern wood lice, sometimes also known as pill-bugs or sow bugs. These are the little bugs (crustaceans, in fact) that are commonly thrown into confusion when you upturn a rock or log.”
- 10:24 PM — Forests are developed and winged insects appear
- 10:55 PM — Dinosaurs appear and stay for 45 minutes
- 11:58 PM — Humans appear. This means that, using this 24-hour clock analogy to look back at the history of this planet and life on Earth, we (humans) have been around for basically a few seconds (2-5 million years). That’s how new we are. It’s mind blowing to think about.
- Quote (P. 337): “Humans emerge one minute and seventeen seconds before midnight. The whole of our recorded history, on this scale, would be no more than a few seconds, a single human lifetime barely an instant.”
- Life On Land: Four Megadynasties — Since life on Earth expanded from water to land, there have been four “megadynasties” that have come about. The creatures that dominated during each of these periods lived for millions and millions of years before giving way to the next megadynasty. Usually extinction events wiped out each phase and led to the next. With humans being so recent, it makes you wonder what is in store for us in the next several million years. Will we eventually become extinct? The megadynasties include:
- Megadynasty 1 — Amphibians & Reptiles
- Megadynasty 2 — Therapsids (Protomammals)
- Megadynasty 3 — Dinosaurs
- Megadynasty 4 — Mammals
- Quote (P. 242): “Each of these massive transformations [megadynasties], as well as many smaller ones between and since, was dependent on that paradoxically important motor of progress: extinction. It is a curious fact that on Earth species death is, in the most literal sense, a way of life. No one knows how many species of organisms have existed since life began. Thirty billion is a commonly cited figure, but the number has been put as high as 4,000 billion. Whatever the actual total, 99.99 percent of all species that have ever lived are no longer with us.”
- Earth, Extinction, and Luck — Throughout the course of life on Earth, extinction has wiped out 99% of all species, and there have been a lot of species (perhaps 30 billion or more). Earth has seen five major extinction episodes in its time — the Ordovician, Devonian, Permian, Triassic, and Cretaceous, in that order — and many smaller ones. The Ordovician (440 million years ago) and Devonian (365 million) each wiped out about 80-85% of species. The Triassic (210 million years ago) and Cretaceous (65 million years) each wiped out 70-75% of species. But the real whopper was the Permian extinction of about 245 million years ago (likely caused by an asteroid), which killed off the dinosaurs. In the Permian, at least 95% of animals known from the fossil record check out, never to return. With all of this in mind, it a remarkable fact that we are here today. In many ways, it’s a miracle. For nearly 4 billion years, our ancestors had to escape and survive some crazy stuff. And they did.
- Quote (P. 349): “We [humans] are so used to the notion of our own inevitability as life’s dominant species that it is hard to grasp that we are here only because of timely extraterrestrial bangs and other random flukes. The one thing we have in common with all other living things is that for nearly four billion years our ancestors have managed to slip through a series of closing doors every time we needed them to.”
- Crazy Creatures — It’s hard to even imagine today, but Earth was once filled with some crazy-looking creatures: Guinea pigs the size of rhinos; raccoons the size of bears. There was even a bird called Titanis that stood 10 feet tall and weighed more than 800 pounds. This bird survived for 50 million years (to put this in perspective, humans have only been around for about 2-5 million years, and modern humans only for a couple thousand years). We only discovered that the Titanis once existed on Earth after finding a skeleton of it in Florida in 1963.
- Quote (P. 347): “For a time, there were guinea pigs the size of rhinos and rhinos the size of a two-story house. Wherever there was a vacancy in the predatory chain, mammals rose (often literally) to fill it. Early members of the raccoon family migrated to South America, discovered a vacancy, and evolved into creatures the size and ferocity of bears. Birds, too, prospered disproportionately. For millions of years, a gigantic, flightless, carnivorous bird called Titanis was possibly the most ferocious creature in North America. Certainly it was the most daunting bird that ever lived. It stood ten feet high, weighed over eight hundred pounds, and had a beak that could tear the head of pretty much anything that irked it.”
Ch. 23: The Richness of Being
- Lots of Life — Between plants, animals, insects, bacteria, and everything else that is alive on our planet, we live among millions and millions of different species, many of which we haven’t even discovered yet. Taxonomists have the job of trying to discover and classify all of these living things, but it’s really difficult and much of the world of living things is still unknown to us. Regarding the number of living things out there in the world, estimates range from 3 million to 200 million, and according the Economist, 97% of the world’s plant and animal species likely haven’t been discovered yet.
- Small Life — One of the major reasons most life forms on Earth remain undiscovered is simply because many organisms are absolutely tiny. Take mites, for example. There are millions of microscopic mites feasting on you at night. Your pillow and blankets are covered in them. These tiny mites weren’t even discovered until 1965 — imagine how many other tiny, tiny living organisms are buried in soil and other nooks and crannies around the world. The planet is absolutely massive, and there simply aren’t enough people hunting for every living thing that roams around.
- Quote (P. 365): “Most living things are small and easily overlooked. In practical terms, this is not always a bad thing. You might not slumber quite so contentedly if you were aware that your mattress is home to perhaps two million microscopic mites, which come out in the wee hours to sup on your sebaceous oils and feast on all those lovely, crunchy flakes of skin that you shed as you doze and toss. Your pillow alone may be home to forty thousand of them. (To them your head is just one large oily bon-bon.) And don’t think a clean pillowcase will make a difference. To something on the scale of bed mites, the weave of the tightest human fabric looks like ship’s rigging. Indeed, if your pillow is six years old — which is apparently about the average age for a pillow — it has been estimated that one-tenth of its weight will be made up of ‘sloughed skin, living mites, dead mites and mite dung’ to quote the man who did the measuring, Dr. John Maunder of the British Medical Entomology Center. (But at least they are your mites. Think of what you snuggle up with each time you climb into a motel bed). These mites have been with us since time immemorial, but they weren’t discovered until 1965.”
Ch. 24: Cells
- About Cells — Cells are the units of life, and we have about 37 trillion of them. Each one carries a copy of our DNA (in the nucleus), along with a bunch of other stuff like proteins, enzymes, ribosomes, and a bunch of other things. The DNA within each cell gives it instructions regarding what to do and how to be. Cells do so many things for us — just about anything you can imagine.
- Quote (P. 372): “Your cells are a country of ten thousand trillion citizens, each devoted in some intensively specific way to your overall well-being. There isn’t a thing they don’t do for you. They let you feel pleasure and form thoughts. They enable you to stand and stretch and caper. When you eat, they extract the nutrients, distribute the energy, and carry off the wastes — all those things you learned about in junior high school biology — but they also remember to make you hungry in the first place and reward you with a feeling of well-being afterward so that you won’t forget to eat again. They keep your hair growing, your ears waxed, your brain quietly purring. They manage every corner of your being. They will jump to your defense the instant you are threatened. They will unhesitatingly die for you — billions of them do so daily. And not once in all your years have you thanked even one of them. So let us take a moment now to regard them with the wonder and appreciation they deserve.”
- Cells: Different Types — We possess a few hundred different types of cells, and they vary tremendously in size and shape. Nerve cells can stretch to several feet, while red blood cells are tiny discs and the cells in our eyes are rod-shaped and help give us vision. Interestingly, our skin cells are completely dead, and we shed several billion of them every day. Much of the dust we see in a house is littered with dead skin cells.
- Quote (P. 373): “Your skin cells are all dead. It’s a somewhat galling notion to reflect that every inch of your surface is deceased. If you are an average-sized adult you are lugging around about five pounds of dead skin, of which several billion tiny fragments are sloughed off each day. Run a finger along a dusty shelf and you are drawing a pattern very largely in old skin.”
- Cells: Routine Death — All of our cells, with the exception of brain and liver cells, last only a month or so. After that, they die and other cells clean up the mess. New cells then swoop in and keep everything running smoothly. This process happens over and over and over again. One exception is brain cells, which last as long as we do. We are born with 100 billion brain cells, and that’s all we ever get. It is estimated that we lose about 500 brain cells every hour. Luckily, the actual internal components of our brain cells replenish constantly, so they are pretty fresh all the time. Liver cells also last much longer than most other cells; they operate for years before dying, but, as with brain cells, their internal parts replenish routinely. When cells don’t die like they’re supposed to, cancer usually arises.
- Quote (P. 373): “Most living cells seldom last more than a month or so, but there are some notable exceptions. Liver cells can survive for years, though the components within them may be renewed every few days. Brain cells last as long as you do. You are issued a hundred billion or so at birth, and that is all you are ever going to get. It has been estimated that you lose five hundred of them an hour, so if you have any serious thinking to do there really isn’t a moment to waste.”
- Quote (P. 373): “The good news is that the individual components of your brain cells are constantly renewed so that, as with the liver cells, no part of them is actually likely to be more than about a month old. Indeed, it has been suggested that there isn’t a single bit of any of us — not so much as a stray molecule — that was part of us nine years ago. It may not feel like it, but at the cellular level we are all youngsters.”
- Cells Are Packed — Interestingly, every one of our cells is absolutely jam-packed with things like a nucleus, proteins, enzymes, a cytoskeleton, and many other components and chemicals. There isn’t any space inside a cell that is left unused. As a result, the activity within a cell is high octane — there is chaos everywhere as proteins are made and enzymes fly around to carry out certain tasks. There’s so much chemical activity going on inside every cell, that actual electricity (albeit a tiny amount) is produced. In this way, you could even say we’re “electrical.”
- Quote (P. 376): “It [a cell] is like a refinery in that it is devoted to chemical activity on a grand scale, and like a metropolis in that it is crowded and busy and filled with interactions that seem confused and random but clearly have some system to them.”
- Quote (P. 376): “There is activity everywhere and a ceaseless thrum of electrical energy [inside a cell]. You may not feel terribly electrical, but you are. The food we eat and the oxygen we breathe are combined in the cells into electricity. The reason we don’t give each other massive shocks or scorch the sofa when we sit is that it is all happening on a tiny scale: a mere 0.1 volts traveling distances measured in nanometers. However, scale that up and it would translate as a jolt of twenty million volts per meter, about the same as the charge carried by the main body of a thunderstorm.”
- Quote (P. 377): “If you could visit a cell, you wouldn’t like it. Blown up to a scale at which atoms were about the size of peas, a cell itself would be a sphere roughly half a mile across, and supported by a complex framework of girders called the cytoskeleton. Within it, millions upon millions of objects — some the size of basketballs, others the size of cars — would whiz about like bullets. There wouldn’t be a place you could stand without being pummeled and ripped thousands of times every second from every direction.”
- Quote (P. 377): “The proteins are especially lively, spinning, pulsating, and flying into each other up to a billion times a second. Enzymes, themselves a type of protein, dash everywhere, performing up to a thousand tasks a second. Like greatly speeded up worker ants, they busily build and rebuild molecules, hauling a piece off this one, adding a piece to that one.”
- Quote (P. 378): “You can see that a cell is just millions of objects — lysosomes, endosomes, ribosomes, ligands, peroxisomes, proteins of every size and shape — bumping into millions of other objects and performing mundane tasks: extracting energy from nutrients, assembling structures, getting rid of waste, warding off intruders, sending and receiving messages, making repairs.”
- Quote (P. 414): “Proteins, you will remember, are the workhorses of all living systems; as many as a hundred million of them may be busy in any cell at any moment.”
- Cells: Mitochondria & ATP — With all of the activity going on inside of every cell, the heart must pump a lot of blood — about 75 gallons an hour! — to keep all of those cells oxygenated. The oxygen is taken in then processed and delivered to the 1,000 or so mitochondria stationed inside of each cell. Mitochondria act like little power stations. The mitochondria convert oxygen and food into a molecule called adenosine triphosphate, or ATP. ATP is what keeps us going. These little molecules act as battery packs that provide us with energy, and they provide our cells with the energy needed to carry out all of their functions. A typical cell will have 1 million ATP molecules, and the cell will burn through those about every two minutes. Another billion ATP molecules will then take their place.
- Quote (P. 378): “Virtually all the food and oxygen you take into your body are delivered, after processing, to the mitochondria, where they are converted into a molecule called adenosine triphosphate, or ATP. You may not have heard of ATP, but it is what keeps you going. ATP molecules are essentially little battery packs that move through the cell providing energy for all the cell’s processes, and you get through a lot of it. At any given moment, a typical cell in your body will have about one billion ATP molecules in it, and in two minutes every one of them will have been drained dry and another billion will have taken their place.”
- Cells: Sending Messages — To add to the impressive features of cells, they somehow communicate with themselves flawlessly. Without their constant messages and communication, we wouldn’t be able to function correctly. There are a couple ways cells send messages. First, cells from various organs (e.g. the thyroid, pancreas, or liver) message other cells around the body via hormones like insulin, adrenaline, estrogen, and testosterone. Second, some messages come down directly from the brain. Third, cells are very good at communicating messages to their direct neighbors.
- Quote (P. 379): “The wonder of cells is not that things occasionally go wrong, but that they manage everything so smoothly for decades at a stretch. They do so by constantly sending and monitoring streams of messages — a cacophony of messages — from all around the body: instructions, queries, corrections, requests for assistance, updates, notices to divide or expire. Most of these signals arrive by means of couriers called hormones, chemical entities such as insulin, adrenaline, estrogen, and testosterone that convey information from remote outposts like the thyroid and endocrine glands. Still other messages arrive by telegraph from the brain or from regional centers in a process called paracrine signaling. Finally, cells communicate directly with their neighbors to make sure their actions are coordinated.”
Ch. 25: Darwin's Singular Notion
- Charles Darwin — Charles Darwin was born February 12, 1809 (the same day as Abraham Lincoln). Darwin is known for a lot of things, but his theory of evolution is by far what he’s most known for. What’s interesting about Darwin is that the idea of evolution had already been a topic of conversation before he was even born. It was when young Darwin read a paper written by Thomas Malthus that he began to form his ideas about evolution and natural selection. His major claim was that organisms adapt to their environments over long periods of time, with those most fit to survive being “selected” to move forward and reproduce, thereby passing their characteristics on to the next generation.
- Darwin: Locking Away the Goods — For various reasons, Darwin actually locked away his theories for 15 years after first developing them. One of the big reasons he did this was due to the prevailing belief system at the time. His theories on evolution flew in the face of Christianity and other religions that believed in a divine creator. He knew his ideas would ignite the world. The only reason he ended up publishing his ideas was because another naturalist named Alfred Wallace developed similar findings and was about to publish them. Darwin caught wind of this and scrambled to put together a presentation of his own. Both Wallace and Darwin ended up unveiling their ideas to the world on the same day — July 1, 1858. Wallace later meandered into other ventures and sort of disappeared. Darwin rose to prominence, particularly thanks to his book On the Origin of Species, which was published in 1859. As a result, Darwin, far more than Wallace, gets all the credit for the idea of evolution.
- Gregor Mendel — Mendel was born in 1822 and is universally recognized for discovering genes, although he never used the word “genes” or “genetics” in any of his writings or presentations. His experiments breeding and crossbreeding hybrids from 30,000 pea plants over eight years led to his discoveries. Amazingly, his work didn’t make much of an impact while he was alive. People just didn’t seem to understand the significance of what he had discovered. It wasn’t until the early-mid-1900s that scientists figured it out.
- Quote (P. 391): “Then, helped by two full-time assistants, he repeatedly bred and crossbred hybrids from thirty thousand pea plants. It was delicate work, requiring them to take the most exacting pains to avoid accidental cross-fertilization and to note every slight variation in the growth and appearance of seeds, pods, leaves, stems, and flowers. Mendel knew what he was doing.”
- Darwin & Mendel — Together but independently, Darwin and Mendel really helped set a foundation for life sciences. At the time, nobody really knew how we became what we are today. Darwin’s theories on evolution, which were completely radical at the time, helped explain that. Nobody knew how evolution was possible, either. Through his discovery of genetics and heredity, Mendel helped answer some of those questions. Remarkably, both lived at the same time but never interacted with each other.
- Quote (P. 393): “Together, without realizing it, Darwin and Mendel laid the groundwork for all of life sciences in the twentieth century. Darwin saw that all living things are connected, that ultimately they ‘trace their ancestry to a single, common source’, while Mendel’s work provided the mechanism to explain how that could happen. The two men could easily have helped each other.”
Ch. 26: The Stuff of Life
- DNA — DNA is your own personal instruction manual stored in the nucleus of every cell in your body. Chromosomes — of which you have 23 from your Mom and 23 from your Dad in the nucleus of every cell — are made of DNA. You have about six feet of DNA located in every cell, and each of those strands comprises 3.2 billion letters of coding. If all of your DNA was woven into a single strand, there would be enough of it to stretch from the Earth to the Moon and back many times.
- DNA: Composition — The shape of DNA is a double helix. DNA has just four basic components that are called nucleotides (also called “bases” or “letters”): adenine (A), cytosine (C), guanine (G), and thymine (T). These are the “rungs” that protrude out from the double helix. The bases always match up with each other: G always goes with C, and T always goes with A. The order in which these letters appear as you move up and down the ladder is what makes up the DNA code.
- DNA & Genes — Amazingly, 97% of our DNA is nothing but meaningless stretches of “highway” that don’t have any impact on us whatsoever. These are “non-coding” stretches of DNA. Some people even call these stretches “junk” because they have no consequences for us. Only once in a while do you run into stretches of code that actually have an impact on us. These stretches (the other 3%) are called genes, and genes are the instructions for making us.
- Quote (P. 408): “Ninety-seven percent of your DNA consists of nothing but long stretches of meaningless garble — ‘junk’, or ‘non-coding DNA’, as biochemists prefer to put it. Only here and there along each strand do you find sections that control and organize vital functions. These are the curious and long-elusive genes.”
- Quote (P. 408): “Genes are nothing more (nor less) than instructions to make proteins. This they do with a certain dull fidelity. In this sense, they are rather like the keys of a piano, each playing a single note and nothing else, which is obviously a trifle monotonous. But combine the genes, as you would combine piano keys, and you can create chords and melodies of infinite variety. Put all these genes together, and you have (to continue the metaphor) the great symphony of existence known as the human genome.”
- DNA: Encoding Proteins — As mentioned earlier, proteins are the hubs of life that carry out so many different functions in our body. DNA (and genes) are what encode these proteins by pumping out chains of amino acids. But DNA and proteins don’t speak the same language — using a language analogy, DNA speaks Spanish and proteins speaks Japanese. So how does DNA communicate its code to make proteins? The answer is RNA, a molecule that essentially clones DNA and interprets the genetic message. It’s the intermediary between DNA and proteins.
- Quote (P. 400): “No one could understand how DNA could possibly be getting messages to the proteins. The answer, we now know, was RNA, or ribonucleic acid, which acts as an interpreter between the two. It is a notable oddity of biology that DNA and proteins don’t speak the same language. For almost four billion years they have been the living world’s great double act, and yet they answer to mutually incompatible codes, as if one spoke Spanish and the other Hindi. To communicate they need a mediator in the form of RNA. Working with a kind of chemical clerk called a ribosome, RNA translates information from a cell’s DNA into terms proteins can understand and act upon.”
- The Same But Different — What’s interesting about us is that, genetically, we are 99.9% the same; it’s the other 0.1% that makes each of us unique. Our genes being 99.9% the same allows us to be a species (human). The 3-4 million places in our DNA where everybody’s genes are different (the 0.1%) are what make us different. These irregularities occur when the four bases of DNA get jumbled and don’t match up correctly. Again, the bases are supposed to always match up with each other: G always goes with C, and T always goes with A. But sometimes this doesn’t happen. Usually, these blemishes happen in the long stretches of DNA that don’t have any impact on us. But sometimes you get a mismatch in one of the stretches of code that does impact us (genes), and those irregularities in nucleotide bases are what make us all different. There are 3-4 million places in our DNA where these irregularities in genes typically occur. These irregularities lead to differences in the way we look, sound, and feel, and they also contribute to certain diseases or predispositions we might encounter.
- Quote (P. 398): “We are also uncannily alike. Compare your genes with any other human being’s and on average they will be about 99.9 percent the same. That is what makes us a species. The tiny differences in that remaining 0.1 percent — ‘roughly one nucleotide base in every thousand’, to quote the British geneticist and recent Nobel laureate John Sulston — are what endow us with our individuality.”
- Quote (P. 409): “Most of the time our DNA replicates with dutiful accuracy, but just occasionally — about one time in a million — a letter gets into the wrong place. This is known as a single nucleotide polymorphism, or SNP, familiarly known to biochemists as a ‘Snip’. Generally these Snips are buried in stretches of noncoding DNA and have no detectable consequence for the body. But occasionally they make a difference. They might leave you predisposed to some disease, but equally they might confer some slight advantage — more protective pigmentation, for instance, or increased production of red blood cells for someone living at altitude. Over time, these slight modifications accumulate in both individuals and in populations, contributing to the distinctiveness of both. . . The 0.1 percent difference between your genes and mine is accounted for by our Snips.”
- All Life Is One — Remarkably, 60% of our genes are the same as a fruit fly’s genes, and 90% of our genes correlate to genes found in mice. Roughly 98.4% of our genes are the same as chimps. And, of course, 99% of my genes are the same as the person next to me. Moreover, we have 35-40,000 genes — about the same number as found in grass. Additionally, about half of the chemical functions that take place in a banana are fundamentally the same as the chemical functions that take place in humans. The point? All life is one. Everything in life is very interconnected.
Ch. 27: Ice Time
- Ice Ages — Ice ages, in which the planet essentially freezes over, have happened several times throughout Earth’s 4.5 billion year history (5 major ones). There are several factors that contribute to an ice age, but one of the most significant is cyclical changes in the Earth’s orbit — when our orbit flattens out a bit and is slightly more circular than elliptical. Rhythmic shifts in Earth’s angle of orientation to the Sun — its tilt, pitch, and wobble — also contribute because they affect the intensity of sunlight falling on any patch of land. When put together, these contributions can lead to cool summers in which snow doesn’t fully melt. When snow doesn’t melt, it builds up and collectively begins to reflect sunlight, enhancing the cooling effect and encouraging more snow. Eventually ice sheets develop and reflect even more sunlight. Over very long periods of time, all of this leads to an ice age.
- Quote (P. 426): “The cause of ice ages, Köppen decided, is to be found in cool summers, not brutal winters. If summers are too cool to melt all the snow that falls on a given area, more incoming sunlight is bounced back by the reflective surface, exacerbating the cooling effect and encouraging yet more snow to fall. The consequence would tend to be self-perpetuating. As snow accumulated into an ice sheet, the region would grow cooler, prompting more ice to accumulate. As the glaciologist Gwen Schultz has noted: ‘It is not necessarily the amount of snow that causes ice sheets but the fact that snow, however little, lasts.’ It is thought that an ice age could start from a single unseasonal summer. The leftover snow reflects heat and exacerbates the chilling effect. ‘The process is self-enlarging, unstoppable, and once the ice is really growing it moves’, says McPhee. You have advancing glaciers and an ice age.”
- Living In an Ice Age — We are currently living in an ice age. The earliest ice age was over 2 billion years ago, and the most recent one (called the Quaternary Glaciation) began approximately 3 million years ago and continues today (yes, we live in an ice age!). During an ice age, the climate fluctuates between glacial periods (cold phases with significant ice sheet coverage) and interglacial periods (warmer phases with reduced ice cover). We are currently in an interglacial period that began about 11,000 years ago. During glacial periods, ice sheets cover large portions of North America, Europe, and Asia. In interglacial periods, such as the one we are in now, these ice sheets retreat significantly.
- Quote (P. 427): “The fact is, we are still very much in an ice age; it’s just a somewhat shrunken one — though less shrunken than many people realize. At the height of the last period of glaciation, around twenty thousand years ago, about 30 percent of the Earth’s land surface was under ice. Ten percent still is — and a further 14 percent is in a state of permafrost. Three-quarters of all the fresh water on Earth is locked up in ice even now, and we have ice caps at both poles — a situation that may be unique in Earth’s history.
- Quote (P. 427): “The current ice age — ice epoch really — started about forty million years ago, and has ranged from murderously bad to not bad at all.”
Ch. 28: Ice Time
- Brief History of Early Humans — For the first 99.9% of our history as organisms, we were in the same ancestral line as chimps. Then, about 7 million years ago, something big happened. A group of new beings emerged from the tropical forests of Africa and began to move about on the open savanna. These were the australopithecines, and for the next 5 million years they were the dominant species. The most famous of these creatures was a one called “Lucy,” who lived 3 million years ago and whose remains were found in Ethiopia in 1974. Lucy is considered our earliest ancestor. She marked the beginning of our evolutionary shift from ape to man. About two million years ago, creatures known as Homo Erectus emerged and began living with the australopithecines. These two species coexisted for a million years before the australopithecines mysteriously vanished.
- Lucy — Lucy was nothing like the modern humans you see today. She was likely no more than 3 feet tall, weighed roughly 60 pounds, and had far fewer bones that we have today. But she was the first of our kind, meaning bipedal creatures. What made these australopithecines like Lucy come down from the trees and out of the forests? They likely had no choice. To survive, they had to come down and start exploring, leading to the next phase of human development.
Ch. 29: The Restless Ape
- Summary of Early Human Life On Earth — This chapter sort of builds on the previous one. It’s mostly about the evolution of early humans. Below is a short summary of the humans that roamed Earth in the early days.
- Australopithecines — For the first 99.9% of our history as organisms, we were in the same ancestral line as chimps. Then, about 7 million years ago, something big happened. A group of new beings emerged from the tropical forests of Africa and began to move about on the open savanna. These were the australopithecines, and for the next 5 million years they were the dominant species. The most famous of these creatures was a one called “Lucy,” who lived 3 million years ago and whose remains were found in Ethiopia in 1974. Lucy is considered our earliest ancestor. She marked the beginning of our evolutionary shift from ape to man.
- Homo Erectus — About two million years ago, creatures known as Homo erectus emerged in Africa and began living with the australopithecines. Eventually, the australopithecines went away forever. Africa therefore is really the origin of human life. Homo erectus left Africa almost immediately after emerging as a species. Over time, they settled in different regions and developed into Homo heidelbergensis and finally Homo neanderthalensis (Neanderthals). Worth noting — these forms of humans were “barely human.” We were still in the process of evolving from our history as chimps and apes.
- Homo Sapiens — Modern humans like us are Homo sapiens, and the first of these humans arrived about 100,000 years ago. That’s it. That is very, very recently when you consider the age of the planet and the age of the first living organisms on Earth. In fact, as Bryson points out, “modern human beings have existed for only about 0.0001% of Earth’s history.” Homo sapiens also first emerged in Africa and the Mediterranean (around modern day Israel) before branching out. Homo sapiens quickly became the dominant species and all others species of human died out.

